


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 29-10-2016
Date: 20-10-2016
Date: 13-10-2016
|
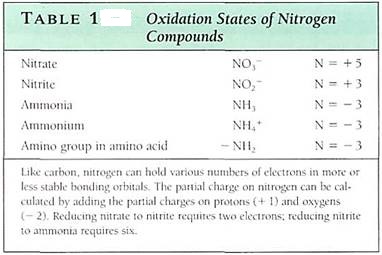
Nitrogen Metabolism
Nitrogen occurs neither as a component of rock matrixes nor as a contaminant in rock; the most abundant source of nitrogen in the environment is the atmospheric gas N2. In this fin; nitrogen is relatively inert chemically and is useless to almost all organisms. To be a useful substrate for most enzymes, it must first be converted to chemically active forms. This process, called nitrogen metabolism, consists of (1) nitrogen fixation, (2) nitrogen reduction, and (3) nitrogen assimilation.
NITROGEN FIXATION
Nitrogen fixation is the conversion of N2 gas into nitrate, nitrite, or ammonium, all forms of ntrogen that are substrates for a variety of enzymes. One means of nitrogen fixation is human manufacturing; the fertilizer industry can synthesize either nitrate or ammonium form atmospheric nitrogen, but this is an extremely expensive and energy-intensive process About 25 million tons of nitrogen fertilizer are produced annually.
Natural processes fix over 150 million tons of nitrogen annually. Lightning is important; the energy of a lightning strike passing through air converts elemental nitrogen to a useful form that dissolves in atmospheric water and falls to the earth in raindrops. How-ever, nitrogen-fixing bacteria and cyanobacteria are by far the most important means of fixing and utilizing atmospheric nitrogen, annually converting 130 million tons of nitrogen to forms that plants and animals can use. These organisms have nitrogenase, an enzyme that can use N2 as a substrate. In a complex reaction, it forces electrons and protons onto; nitrogen, reducing it from the +0 oxidation state to the —3 oxidation state (Table 1). Ammonia, NH3, is the product; it immediately dissolves in the cell's water and picks up a proton, becoming ammonium, NH4+. Nitrogenase is a giant enzyme or enzyme complex; it has a molecular weight of 100,000 to 300,000 daltons and contains 15 to 20 iron atoms and 1 or 2 molybdenum atoms. It is extremely sensitive to oxygen and functions only if oxygen is completely excluded from it.
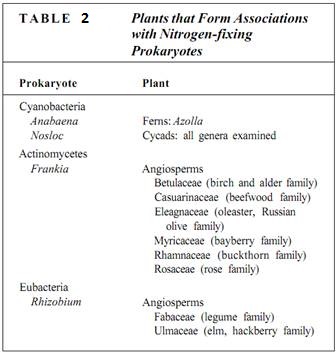
Some nitrogen-fixing microorganisms are free-living in the soil; examples are Azotobarter, Clostridium, Klebsiella, and some cyanobacteria like Nostoc (Fig. 1). The nitrogen that they fix is used in their own metabolism and becomes available to plants and fungi only when they die and their bodies decay. Other nitrogen-fixing organisms live symbiotically, growing inside the tissue of host ferns and seed plants (Table 2). The best-known examples are the root nodules on legumes such as alfalfa; the nodules are growths of root tissue whose cells contain bacteria of the genus Rhizobium.

FIGURE 1:Cyanobacteria are common components of most soils, although usually they are quite inconspicuous. This species of Nostoc forms large masses (a) easily visible to the naked eye and covering large areas of the soil. They swell and fix nitrogen rapidly when wet (a), but they become dormant and crisp when dry (b). Even though extremely desiccated with an extraordinarily negative water potential, the cells are alive and revive within seconds of receiving water.
Plants such as alders (Alnus), bog myrtle (Myrica gale), and Casuarina equisetifolia are pioneer plants that are the first to grow in poor, nitrogen- deficient soils such as bogs, sand dunes, and glacial rubble. These species obtain their nitrogen from a symbiosis with the prokaryote Frankia. The symbiotic bacterial cells use part of the fixed nitrogen for their own growth and reproduction, but they also permit large amounts to leak out into the protoplasm of the surrounding root cells. Symbiotic nitrogen-fixes usually produce fixed nitrogen at a much greater rate than free-living microorganisms, perhaps because they have the energy resources of the plant at their disposal.
The rate at which plant/prokaryote symbioses fix nitrogen is strongly influenced by the stage of development of the plant: When soybeans begin to produce their protein-rich safe (40% protein, the richest seeds known), nitrogen fixation in the roots increases greatly. As much as 90% of all nitrogen fixation occurs during the phase of seed development, whereas only 10% occurs during all the vegetative growth that precedes it. Furthermore;; if legume crop plants are given high levels of nitrogen fertilizer, plants that have not yet formed nodules do not produce them, and those that already have bacteroid-filled nodules decrease the amount of nitrogen fixed and even allow the nodule to senesce. With adequate nitrogen available in the environment, it is selectively advantageous not to pass glucose on to bacteria.
NITROGEN REDUCTION
Nitrogen reduction is the process of reducing nitrogen in the nitrate ion, NO3", from an oxidation state of +5 to the —3 oxidation state of ammonium, which is also the oxidation state of nitrogen in amino acids, nucleic acids, and many other biological compounds . Nitrogenase automatically reduces nitrogen during the fixation process, and if plant can absorb that form of nitrogen, no further reduction is necessary. Also, as organic matter decays in the soil, ammonium is released and becomes available. Unfortunately for plants ammonium is an extremely energetic compound that numerous species of soil bacteria can use as "food," oxidizing it to produce ATP . In the process, ammonium is converted to nitrate. Such soil bacteria are so common that ammonium lasts ay a short time in the soil, and the predominant form of nitrogen available to roots is the oxidized form, nitrate.
Reducing nitrate back to ammonium requires eight electrons for each nitrogen atom and a great deal of energy. In the first step, two electrons are added, reducing nitrogen form +5 to +3 and forming nitrite, NO2-. The enzyme is nitrate reductase, and it carries electrons by means of a molybdenum atom (Fig. 2). Just like electron carriers in photosynthesis or respiration, when nitrate reductase reduces nitrate, it becomes oxidized and must pick up more electrons. It gets these from FADH2 and NADH; the ultimate source of energy and electrons is respiration.
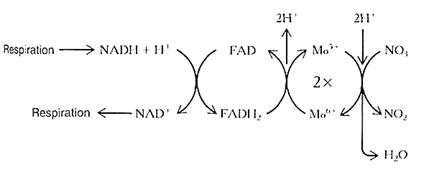
FIGURE 2:The electrons that reduce nitrate to nitrite are brought to it by a short electron transport chain. The FAD and molybdenum are actually bound to the nitrate reductase enzyme, but NADH and NAD+ diffuse between the enzyme and sites of respiration.
In the second step, nitrite reductase adds six electrons to nitrite, reducing it to ammonium. The process is not well understood, but it is known that extremely strong reducing agents are needed. Apparently, nitrite reduction in leaves is powered by reduced ferredoxin from the light reactions of chloroplasts, but roots are a more important site of nitrite reduction, and of course they have no light reactions. Instead they use NADPH, which is produced by the pentose phosphate pathway. Although ATP is not consumed during nitrogen reduction, the process is expensive energetically because the NADH, NADPH, and ferredoxin used are no longer available for ATP synthesis in the mitochondrial electron transport chain.
In leaves, nitrate and nitrite are present as a result of breakdown of amino adds,] nucleic acids, and other nitrogenous compounds during normal metabolism; nitrogen reduction recycles the nitrogen and conserves it within the plant.
NITROGEN ASSIMILATION
Nitrogen assimilation is the actual incorporation of ammonium into organic molecules in the plant body. The process is similar to that of an electron transport chain: Reduced nitrogen passes through a series of carriers that function repeatedly but in the long run are not changed.
The acceptor molecule is glutamate (the ionized form of glutamic acid); it reacts with ammonium and ATP, producing glutamine and ADP (Figs. 3 and 4). Glutamine transfers the ammonium, now referred to as an amino group, to alpha-ketoglutarate transforming both molecules into glutamate. The original acceptor has been regenerated, and an extra molecule of glutamate has been produced which can in turn transfer its amino group to another molecule. If the next molecule to receive the amino group is oxaloacetate, the amino acid aspartate is produced. If pyruvate receives the amino group, the amino acid alanine is produced.
These glutamate-mediated transfers of amino groups are the bases for incorporating nitrogen into the plant's metabolism and synthesizing all the amino acids, nucleotides, chlorophyll, and many more compounds. The transfer of an amino group from one molecule to another is transamination.
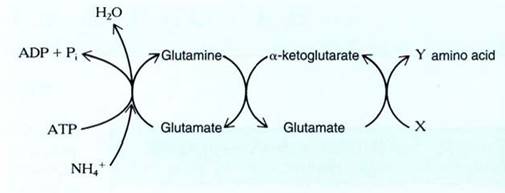
FIGURE 3 :Nitrogen assimilation occurs by means of an "amino transport chain." The acceptor molecule is glutamate, which is already an amino acid, having one amino group. When it picks up ammonia, it becomes an amine, having two amino groups. One is passed on to alpha-ketoglutarate, regenerating the acceptor and producing a new carrier. The next step is variable: By using various acids at X, the cell produces different amino acids at Y. If oxaloacetate is used, the amino acid aspartate results.
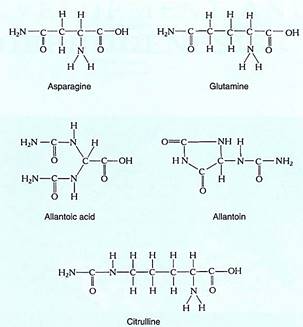
FIGURE 4:These chemicals each have a high nitrogen-to-carbon ratio and are transport forms of reduced nitrogen. Once moved through the phloem to a nitrogen sink, the compounds are catabolized and the amino group is used in the synthesis of amino acids, nucleic acids, and other compounds.
Nitrogen assimilation usually occurs in roots, the site of either absorption of nitrate or ammonium or transfer of ammonium from symbiotic prokaryotes. Much of the assimilated nitrogen must be transported to the shoot through the vascular tissues. Several nitrogen- rich compounds arc common: Asparagine and glutamine both contain two amino groups rather than just one, as in the corresponding amino acids (Fig. 13.18). In legumes, allantoic acid and allantoin are common transport forms, and in alders, nitrogen is carried from roots as citrulline.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|