


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-5-2016
Date: 31-8-2017
Date: 13-2-2016
|
BUTYLENES
Butylenes (butenes) are by-products of refinery cracking processes and steam cracking units for ethylene production. Dehydrogenation of butanes is a second source of butenes. However, this source is becoming more important because isobutylene (a butene isomer) is currently highly demanded for the production of oxygenates as gasoline additives. There are four butene isomers: three unbranched, “normal” butenes (n butenes) and a branched isobutene (2-methylpropene). The three nbutenes are 1-butene and cis- and trans- 2-butene. The following shows the four butylene isomers:
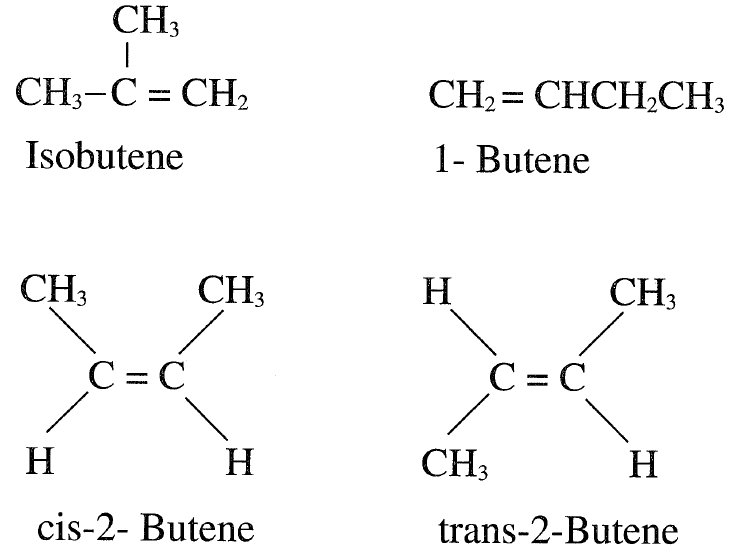
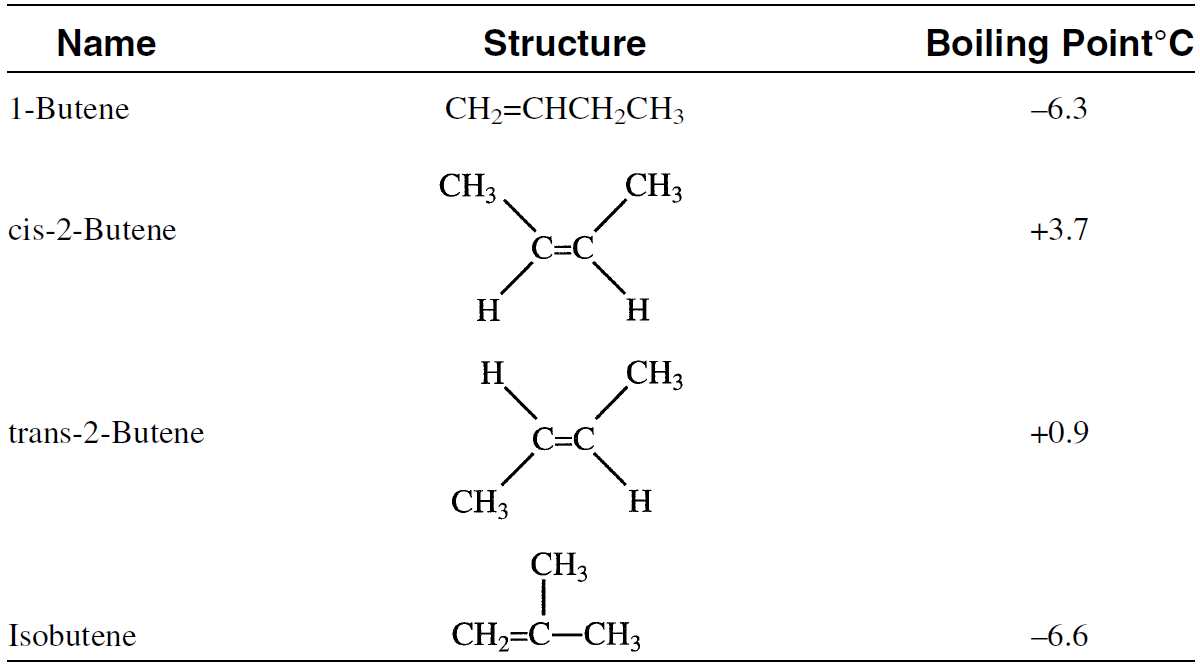
The industrial reactions involving cis- and trans-2-butene are the same and produce the same products. There are also addition reactions where both l-butene and 2-butene give the same product. For this reason, it is economically feasible to isomerize 1-butene to 2-butene (cis and trans) and then separate the mixture. The isomerization reaction yields two streams, one of 2-butene and the other of isobutene, which are separated by fractional distillation, each with a purity of 80–90%. Table 1-1 showst he boiling points of the different butene isomers.
Table 1-1: Structure and boiling points of C4 olefins6
An alternative method for separating the butenes is by extracting isobutene (due to its higher reactivity) in cold sulfuric acid, which polymerizes it to di- and triisobutylene. The dimer and trimer of isobutene have high octane ratings and are added to the gasoline pool. Figure 1 shows the two processes for the separation of n-butenes from isobutene.
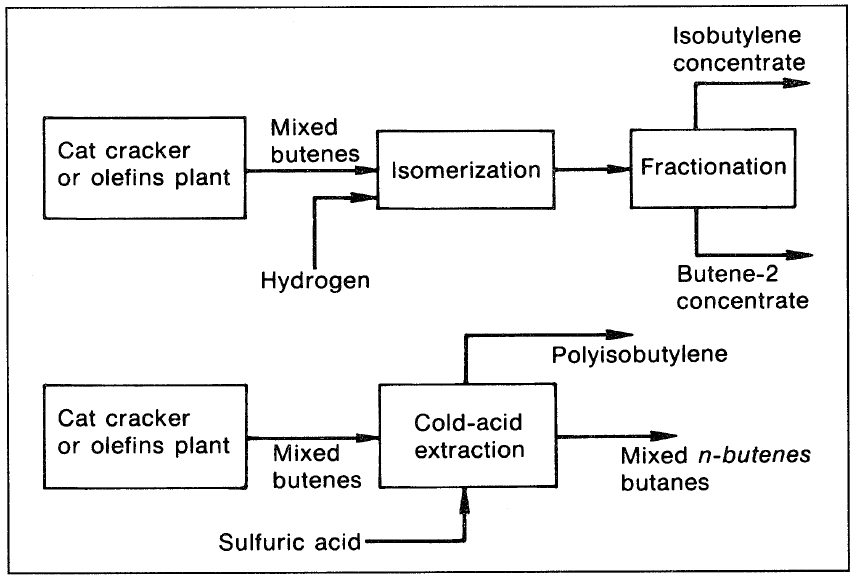
Figure 1. The two processes for separating n-butenes and isobutylene



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|