


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 8-3-2016
Date: 8-3-2016
Date: 27-12-2020
|
FRANCK HERTZ EXPERIMENT
The Franck–Hertz experiment demonstrates the fact that energy levels in atoms are indeed quantized into discrete levels. We have assumed this right from the start, beginning with the process of emission of light, and it is a foundation for the quantum approach to explaining light emission. It is considered to be one of the great experiments in physics. Although the original experiment by Franck and Hertz used mercury vapor, it is more convenient to use neon, since it provides visible evidence of these levels. The experiment, shown in Figure 1.1, consists of a gas-filled vacuum tube (i.e., gas at low pressure) with a heated cathode that emits electrons into the gas. These electrons are accelerated toward a grid at a more positive potential than the cathode. This potential is adjustable, allowing the experimenter to give accelerated electrons a specific energy. Electrons then pass through the grid and are collected at the anode, where they show up as current (electron flow is, by definition, electric current). As the voltage between the cathode and the grid is increased, electrons emitted from the hot cathode are accelerated through the gas and toward the grid. As expected, a rising current is seen at the anode, which collects electrons traveling through the tube. When the accelerating voltage reaches about 18.7 V, the anode current suddenly drops. As the accelerating voltage increases, the current begins to rise again, until the voltage reaches 37.4 V, where another drop occurs. When current through the gas is plotted against accelerating potential, a series of dips in collector current occur at periodic intervals, as shown in Figure 1.2. Dips (with corresponding peaks) in collector current are seen at intervals of 18.7 V, as expected. Even more interesting are bands of light and dark that appear in the tube, as evident in Figure 1.3. At an accelerating voltage of about 40 V, two bands of light appear as evident in part (a), and when the accelerating voltage is about 60 V, three bands of light appear, as shown in part (b). Examining the situation, we find that by definition the accelerated electrons have an energy of 18.7 eV at the point where the first dip occurs, an electron volt being defined as the energy an electron has when accelerated across a potential of 1 V.
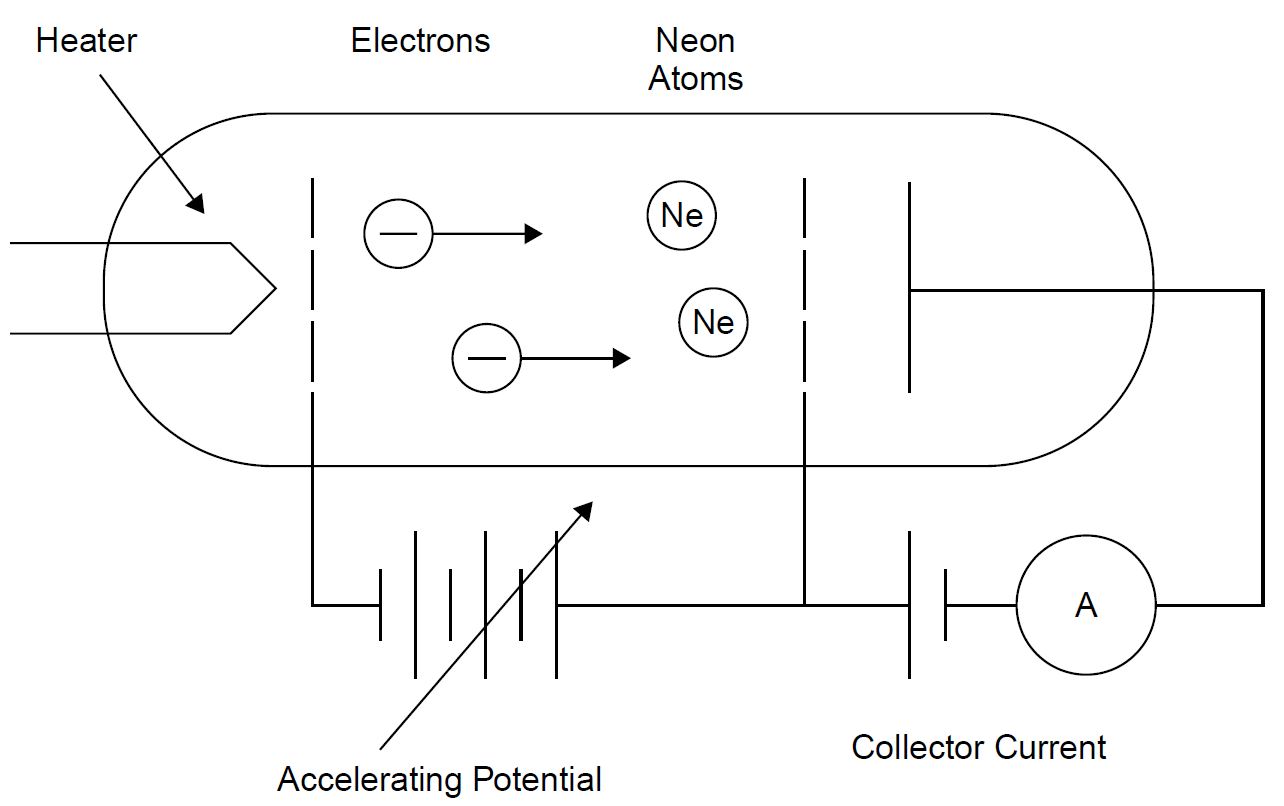
Figure 1.1. Franck Hertz experiment setup.
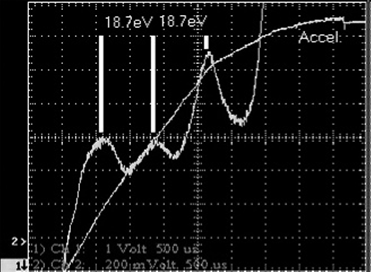
Figure 1.2. Franck Hertz observations: collector current.
This dip corresponds to a known energy level in the neon atom, the first such level above ground. The interpretation of this experiment is that electrons below 18.7 eV do not have enough energy to excite the neon atom to an allowed energy and so pass

Figure 1.3. Franck–Hertz observations: visual emissions.
through the gas unimpeded, being manifested as current through the tube (i.e., electron flow). At 18.7 eV the electrons have enough energy to excite neon atoms to their first excited level upon impact. From basic kinematics we see this as the inelastic collision of the lightweight, excited electron with the more massive neon atom. The result of the collision is that the energy of the electron is totally absorbed by the much heavier atom, pushing the neon atom’s energy to the first excited state. The surprising part is that the energy of the neon atom is quantized it can only take on certain allowed values. Electrons whose energy is below 18.7 eV cannot transfer energy to the neon atom to excite it. The corresponding drop in current occurs because electrons at that energy are no longer flowing though the tube to the anode (i.e., current flow) but rather, are transferring their energy to neon atoms (where they show up as emitted light). It is evident from Figure 1.2 that when the accelerating voltage is ramped from zero to 70 V, three dips in current occur. Electrons emitted from the heater (filament) travel down the tube and acquire energy. Until they reach 18.7 eV, they cannot transfer energy to the abundant neon atoms and so travel unimpeded. When the electrons have 18.7 eV of energy, a good number of them will collide with neon atoms and transfer energy to them. These neon atoms will acquire the energy but will eventually lose it by emitting a photon and returning to ground level (this is actually a two-step process in neon). Electrons, now depleted in energy by the collision, will continue down the tube acquiring more energy until they again reach 18.7 eV, at which point they can again collide with neon atoms and transfer energy to them. The process continues until the electrons reach the anode. With a 70-V accelerating potential, we would expect three dips as well as three corresponding areas in the tube where neon atoms emit light, as shown in Figure 1.3(b). In Figure 1.3, electrons in the dark space just above the lower grid are accelerating upward away from that grid toward the top of the tube. They lack sufficient energy to excite neon’s energy levels and so retain their energy as they travel down the tube. Eventually, moving upward as they acquired energy, they possess enough energy to excite neon’s first energy level, so collisions with neon atoms will result in energy transfer. Excited neon atoms emit light (the lowest band of orange light between the grids in the photo) and in doing so lose energy. Still moving upward, we see a lack of light emission as low-energy electrons travel through, acquiring more energy due to the applied potential. By the time the electrons reach the area of the second band of light, they have again acquired the necessary energy to excite neon’s first level, so light emission occurs again as electrons transfer their energy to neon atoms in this region. In Figure 1.3(b) the process repeats again, with electrons acquiring energy a third time to transfer to neon atoms by collisions (the third band of light), until they finally reach the top grid and stop accelerating. The Franck Hertz experiment demonstrates that atoms do indeed have quantized, discrete energy levels. These levels define the excitation of the atom. An atom that is not excited (and hence is in its lowest-energy configuration) is said to be at ground state. As energy is absorbed by the atom, it is elevated to higher-energy states. In the case of neon, the first excited state is observed to be at about 18.7 eV4. For hydrogen, the first excited state is observed to be at 3.4 eV above ground state.



|
|
|
|
إجراء أول اختبار لدواء "ثوري" يتصدى لعدة أنواع من السرطان
|
|
|
|
|
|
|
دراسة تكشف "سببا غريبا" يعيق نمو الطيور
|
|
|
|
|
|
اللجنة التحضيرية للمؤتمر الحسيني الثاني عشر في جامعة بغداد تعلن مجموعة من التوصيات
|
|
|
|
السيد الصافي يزور قسم التربية والتعليم ويؤكد على دعم العملية التربوية للارتقاء بها
|
|
|
|
لمنتسبي العتبة العباسية قسم التطوير ينظم ورشة عن مهارات الاتصال والتواصل الفعال
|
|
|
|
في جامعة بغداد.. المؤتمر الحسيني الثاني عشر يشهد جلسات بحثية وحوارية
|