


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 4-4-2021
Date: 10-12-2015
Date: 14-12-2015
|
Cauliflower Mosaic Virus
Cauliflower mosaic virus (CaMV) was the first plant virus shown to have a DNA genome and the first shown to replicate by reverse transcription. The virus is found worldwide but only causes significant losses locally. It is transmitted by aphids in the externally borne or stylet-borne manner and encodes a helper factor that is required for transmission. It has a narrow host range, being restricted primarily to the Cruciferae. CaMV is the type member of the Caulimovirus genus, which contains 11 species and 6 possible members.
This virus has had a significant impact on plant virology and plant molecular biology. The unravelling of the reverse transcription replication mechanism led to the concept of pararetroviruses (1, 2) .The virus is an important source of gene regulatory elements, which have been used extensively in the genetic manipulation of plants.
1. Virus Structure
CaMV particles are isometric, about 50 nm in diameter (Fig. 1). Although the virus particles have been crystallized, X-ray crystallography did not yield detailed information, most probably because of the heterogeneity of the coat protein (3) The structure has been shown by cryoelectron microscopy and three-dimensional image reconstruction to comprise three concentric layers of solvent-excluded density (4). The outermost layer is made up of a total of 420 coat protein subunits arranged in T = 7 icosahedral symmetry; it is the first example of a T = 7 virus that obeys the stoichometric rules of icosahedral symmetry. The DNA genome is distributed in layers II and III, together with some of the viral coat protein. The viral coat protein is expressed from open reading frame IV as a 58-kDa precursor molecule that is processed proteolytically to several molecular sizes, the major ones being 42 and 37 kDa. The protein in virions is glycosylated and phosphorylated, and the virions also contain minor amounts of the products of open reading frames III and V.
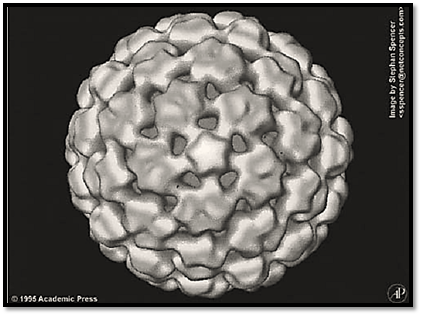
Figure 1. External structure of cauliflower mosaic virus, viewed down a local five-fold axis.
CaMV particles are very stable and are only dissociated at high pH (5) or by digestion of the coat protein with proteinases.
2. Viral Genome
Each virus particle contains a single molecule of circular double-stranded DNA of 8 kbp. The DNA of most isolates of CaMV has three discontinuities (D1, D2, and D3 in Fig. 2), one in one strand (the a strand) and two in the other (yielding the b and g strands); one strain has only two discontinuities, one in each strand. The discontinuities have an unusual structure, with a fixed 5′ DNA nucleotide (with sometimes one or two ribonucleotides attached) and variable 3′ end, overlapping the 5′ end by 10 to 30 nucleotides. They are the sites of the priming of (+)- and (–)-strand DNA replication, and this unusual structure results from viral replication (see below). The DNA has a twisted conformation that, because of the discontinuities, cannot be supercoiled, the constraining forces being unknown.
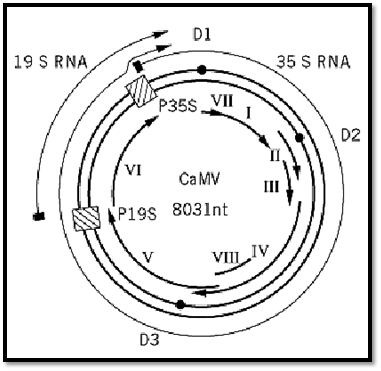
Figure 2. Genome organization of CaMV. The double-stranded DNA genome is represented by the thick double circle, with the discontinuities shown as ● . The promoters for the 35S and 19S transcripts are indicated by  , and the positions of the transcripts are shown by the outer arcs. The inner arcs are the open reading frames I to VIII.
, and the positions of the transcripts are shown by the outer arcs. The inner arcs are the open reading frames I to VIII.
CaMV DNA has six, or possibly eight, open reading frames (ORFs) on the complement to the a strand (Fig. 2). ORF I product (37 kDa) is involved in the cell-to-cell movement of the virus, and ORF II product (18 kDa) is the aphid transmission protein (see Virus Infection, Plant). The product of ORF III is a protein of 15 kDa that is processed to 11 kDa and thought to be involved in packaging the DNA genome (6). ORF IV encodes the viral coat protein, which contains the “cys” motif Cys– X2Cys– X4 His– X4Cys– characteristic of retrovirus gag or nucleoproteins and a highly basic lysine-rich region. The viral polymerase (reverse transcriptase +ribonucleaseH) is encoded by ORF V (79 kDa). The product of ORF VI forms the matrix of the inclusion bodies in which virus particles are found embedded in the cytoplasm of infected cells. It has several functions, including being the site of the reverse transcription phase of viral replication (7), involved in virus assembly (8), a transactivator during viral expression (see below), and a symptom determinant. No products have been found for ORFs VII and VIII, and it is uncertain whether they are expressed.
3.Transcription and Translation
CaMV DNA is transcribed asymmetrically from the a strand to give a more-than-genome length transcript (the 35S RNA), with a terminal repeat of 180 nucleotides and subgenomic transcript (the 19S RNA) that is the messenger RNA for ORF VI (Fig. 2); the transcripts are polyadenylated. There are suggestions of other transcripts, such as one for ORF V (9), but these have not been characterized. Splicing events between the leader sequence and an acceptor site in ORF II (10) are essential for virus infectivity. It is possible that these events might form the mRNA for expression of ORF III and, possibly, ORFs IV and V.
The promoter for the 35S RNA is well characterized and widely used in the expression of transgenes in plants. It directs constitutive, high-level expression in most tissues of most plant species. The promoter has a modular structure, with regions that control the tissue specificity of expression [reviewed in 11). Nuclear factors have been identified that bind to specific regions of the promoter. The 19S promoter is much weaker than the 35S promoter and is less well characterized.
The CaMV genome also contains the signal sequence for the polyadenylation of both the 35S and 19S transcripts. Due to the terminal repeat, the transcription of the 35S RNA has to pass this signal the first time it is encountered and recognize it the second time. It is thought that the bypass is due to the proximity of the promoter (12).
Translation of the 35S RNA is effected by several unusual mechanisms [reviewed in (11) and further refined in (10)). The leader sequence upstream of ORFI is long (>600 nucleotides) and contains several AUG start codons, which could inhibit downstream translation. However, this leader sequence is folded into a complex stem-loop structure, which enables small ribosomal subunits to “shunt” from the 5′ to 3′ end of the leader sequence without scanning the sequence in between; this then opens up ORFI. Translation of downstream ORFs on this polycistronic message is dependent on the presence of a transactivator, which is the product of ORF VI, and possibly on the splicing mechanism described above. This process of transactivation seems to enable ribosomes that have translated one ORF to remain competent to translate the next ORF downstream.
4. Viral Replication
The replication cycle of CaMV (Fig. 3) has been studied in much detail, but there are are still several aspects that are not fully understood. Virus particles enter the uninfected cell and are unencapsidated by an unknown mechanism. The viral DNA genome enters the nucleus, where the discontinuities are sealed and the molecule associates with histones forming a minichromosome (Fig. 3, step 2). This is the template for transcription by the host DNA-dependent RNA polymerase, giving the 35S and 19S RNAs, which pass to the cytoplasm (Fig. 3, step 3). The 19S RNA is translated to yield the inclusion body protein. The 35S RNA has two functions, being the mRNA for at least the products of ORFs I and II and the template for the reverse transcription phase of replication. There is some evidence that this phase of replication takes place in viruslike particles in the inclusion bodies (7). The first event in reverse transcription is the annealing of the 3′ end of tRNAmetinit to a site, that of discontinuity 1 ) D1), about 600 nucleotides from the 5′ end of the 35S RNA (Fig. 3 step 4). This acts as a primer for the synthesis of a-strand DNA toward the 5′ end of the template. RNaseH activity removes the RNA moiety of the DNA–RNA duplex thus formed and leaves DNA (Fig. 3, step 5) that, because of the terminal repeat of the 35S RNA, is complementary to the 3′ end of the template. Thus, a strand switch is effected, and synthesis of the a strand DNA continues (Fig. 3, step 6). The digestion of the RNA by RNaseH leaves purine-rich regions at the positions of discontinuities 2 and 3 (D2 and D3), which act as primers for the synthesis of the b and g strands (Fig. 3 steps 7 and 8). When the oncoming strand of (+)-strand DNA reaches the next priming site, it displaces the primer and some newly synthesized DNA, resulting in the triple-strand discontinuity described above. A second strand switch occurs at the tRNA priming site between the oncoming a and g strands, allowing the synthesis of the g strand to continue and making discontinuity 1.
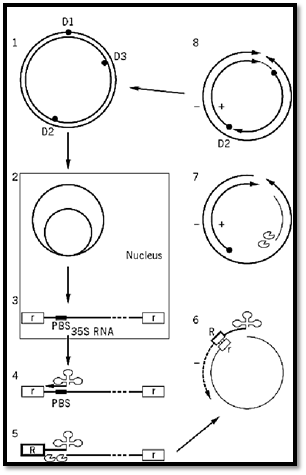
Figure 3. Replication cycle of CaMV. Step 1: The input DNA containing discontinuities 1–3 (D1–D3) passes to the nucleus where it forms minichromosomes (step 2). These are the template for the transcription of the 35S RNA (step 3(with terminal repeats  , which moves to the cytoplasm. Priming of the 35S RNA occurs by the annealing of the 3′ end of tRNAmetinit
, which moves to the cytoplasm. Priming of the 35S RNA occurs by the annealing of the 3′ end of tRNAmetinit 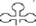 to the primer binding site (PBS) on the 35S RNA and leads to the synthesis of (–)-strand DNA (step 4). RNaseH removes the RNA of the RNA DNA duplex, leaving the complement to the terminal repeat sequence
to the primer binding site (PBS) on the 35S RNA and leads to the synthesis of (–)-strand DNA (step 4). RNaseH removes the RNA of the RNA DNA duplex, leaving the complement to the terminal repeat sequence  (step 5). This anneals to the 3′ end of the 35S RNA, and DNA synthesis continues (step 6). RNAseH activity leaves purine-rich regions
(step 5). This anneals to the 3′ end of the 35S RNA, and DNA synthesis continues (step 6). RNAseH activity leaves purine-rich regions  , which act as primers for (+)-strand DNA synthesis (steps 7 and 8). The oncoming strand displaces the primer sequence, thus giving the characteristic discontinuities, and a second strand-switch is effected at D1 to complete the molecule.
, which act as primers for (+)-strand DNA synthesis (steps 7 and 8). The oncoming strand displaces the primer sequence, thus giving the characteristic discontinuities, and a second strand-switch is effected at D1 to complete the molecule.
References
1. R. Hull and H. Will (1989) Trends Genet. 5, 357–359.
2. H. Temin (1989) Nature 339, 254–255.
3. Z. X. Gong, H. Wu, R. H. Cheng, R. Hull, and M. G. Rossmann (1990) Virology 179, 941–945.
4. R. H. Cheng, N. H. Olson, and T. S. Baker (1992) Virology 186, 655–668.
5. R. Al Ani, P. Pfeiffer, G. Lebeurier, and L. Hirth (1979) Virology 93, 175–187.
6. J. L. Mougeot, T. Guidasci, T. Wurch, G. Lebeurier, and J. M. Mesnard (1993) Proc. Natl. Acad. Sci. USA 90, 1470–1473.
7. C. M. Thomas, R. Hull, J. A. Bryant, and A. J. Maule (1985) Nucl. Acids Res. 13, 4557–4576.
8. A. Himmelbach, Y. Chapdelaine, and T. Hohn (1996) Virology 217, 147–157.
9. A. L. Plant, S. N. Covey, and D. Grierson (1985) Nucl. Acids Res. 13, 8305–8321.
10. Z. Kiss-László, S. Blanc, and T. Hohn (1995) EMBO J. 14, 3552–3562.
11. H. M. Rothnie, Y. Chapdelaine, and T. Hohn (1994) Adv. Virus Res. 44, 1–67.
12. H. Sanfaçon and T. Hohn (1990) Nature (Lond.) 346, 81–84.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|