


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 6-11-2020
Date: 28-12-2015
Date: 16-12-2015
|
Caspases
Caspases are proteinases involved in apoptosis and programmed cell death. They are mammalian homologues of the Ced-3/interleuking-1b converting enzyme (ICE) products of cell death genes (1). The caspase family in mammalian cells currently numbers 14, as shown in Figure 1. They are thiol proteinases that are synthesized as inactive pro-proteins that are processed to a pro-domain and two subunits of approximately 10 and 20 kDa in size. The pro-caspases are inert until proteolytic cleavage of the pro-domain and/or the p10 subunit from the p20 subunit. Caspases share a conserved active-site sequence, -Gln-Ala-Cys-Arg-Gly- (QACRG) or -Gln-Ala-Cys-Gln-Gly- (QACQG), and cleave their substrates at the peptide bond after an aspartate residue; hence, their name- cysteine aspartate-specific proteinases (1, 2). Caspases cleave specific substrates during apoptosis and are required for the rapid degradation of the cell (2-6); however, none is thought to be critical for apoptosis to occur.
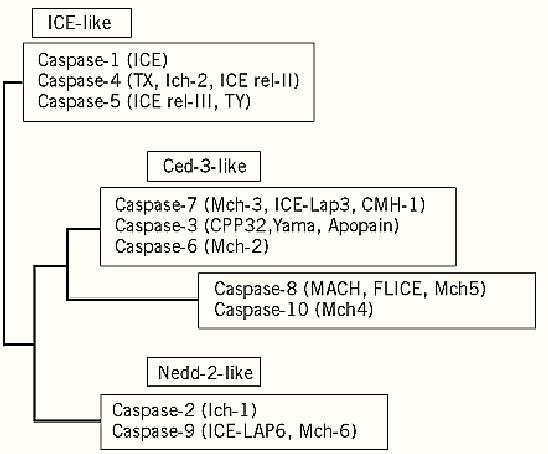
Figure 1. The mammalian caspase family. The various members have been arranged in a phylogeny on the basis of their relative amino acid sequence homologies. The members of the Ced-3-like family of caspases are thought to be the downstream effectors of apoptosis and to be activated in a caspase cascade by the apical members represented by caspases 2, 8, 9, and 10 (1).
Caspases can be divided into subgroups, with each sharing a specific substrate specificity beyond the overall caspase requirement for Asp at the P1 position of the substrate (see Proteinases). Caspases 1, 4, and 5, for example, preferentially cleave after -Tyr-Val-Ala-Asp- (YVAD) sequences and have not been shown to be critical for apoptosis to occur. Caspases 3 and 6, however, are active during cell death and cleave primarily substrates with the sequence -Asp-Xaa-Xaa-Asp- (DxxD). Caspases 2, 8, and 10 are different again and are thought to activate primarily the downstream caspases, such as caspase 3 and 6 (7). This suggests that caspases act in a hierarchy, with activated upstream caspases cleaving downstream caspases and activating them in turn, to generate a caspase cascade (4, 8) .The apical caspases are proposed to be those with large pro-domains such as caspases 2, 8 and 10. The pro-domains contain a protein structure that facilitates their interaction with other proteins leading to caspase activation, as seen in CD95 (Fas/Apo-1)-induced apoptosis (9).
References
1. E. Alnemri, D. Livingston, D. Nicholson, G. Salvesan, N. Thornberry, W. Wong, and Yuan, J. (1996) Human ICE/CED-3 protease nomenclature. Cell 87, 171.
2. A. Takahashi and W. Earnshaw (1996) Ice-related proteases in apoptosis. Curr. Opin. Gen. Dev. 6, 50-55 .
3. A. Chinnaiyan and V. Dixit (1996) The cell-death machine. Curr. Biol. 6, 555–562.
4. Y. Lazebnik, A. Takahashi, G. Poirier, S. H. Kaufmann, and W. Earnshaw (1995) Characterization of the execution phase of apoptosis in vitro using extracts from condemned phase cells. J. Cell. Sci. 19, 41–49.
5. M. Whyte (1996) ICE/Ced-3 proteases in apoptosis. Trends Cell Biol. 6, 245–248.
6. A. Fraser, N. McCarthy, and G. I. Evan (1996) Biochemistry of cell death. Curr. Opin. Neurobiol. 6, 71–80.
7. T. Fernandes Alnemri, R. C. Armstrong, J. Krebs, S. M. Srinivasula, L. Wang, F. Bullrich, L. C. Fritz, J. A. Trapani, K. J. Tomaselli, G. Litwack, and E. S. Alnemri (1996) In vitro activation of CPP32 and Mch-3 by Mch-4, a novel human apoptotic cysteine protease containing 2 FADD-like domains. Proc. Natl. Acad. Sci. USA 93, 7464–7469.
8. Y. A. Lazebnik, A. Takahashi, R. D. Moir R. D. Goldman, G. G. Poirier, S. H. Kaufmann, and W. C. Earnshaw (1995) Studies of the lamin proteinase reveal multiple parallel biochemical pathways during apoptotic execution. Proc. Natl. Acad. Sci. USA 92, 9042–9046.
9. A. Fraser and G. Evan (1996) A license to kill. Cell 85, 781–784.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|