


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 21-3-2021
Date: 5-6-2021
Date: 25-4-2016
|
Brefeldin A
Brefeldin A (BFA) is a fungal metabolite that is a powerful tool for dissecting membrane traffic and organelle dynamics. It is named after Penicilium brefeldianum, which produces it. BFA is a macrolide antibiotic with a molecular weight of 280.36 Da and the structure
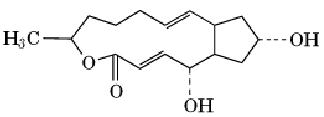
BFA is normally used on cells in culture at concentrations of 1 to 10 µM. As first discovered by Misumi et al. (1), BFA primarily inhibits secretion, without significantly affecting endocytosis. It does not affect protein biosynthesis significantly, does not deplete cellular ATP levels, and does not affect cytoskeletal elements. The unique feature of BFA, which distinguishes it from other inhibitors of protein traffic, is that it causes coalescence of organelles. Within minutes of BFA application, the Golgi apparatus fragments and fuses with the endoplasmic reticulum (ER), which does not fragment (2) leading to mixing of the two compartments.
The effects of BFA are completely reversible. Retrograde movement of Golgi proteins into the ER occurs via long, tubulovesicular processes extending out of the Golgi along microtubules. This retrograde traffic pathway can be separated into two distinct phases: movement of Golgi proteins into the intermediate compartment between the ER and Golgi complex, followed by cycling between the modified ER and the intermediate compartment (3). Remarkably, many Golgi enzymes are still active in this environment and can modify proteins in the ER (4).
One of the important uses of BFA-induced redistribution has been the functional demarcation of compartments along the secretory pathway (5). It is clear that BFA affects the early Golgi compartments, the cis and medial cisternae. Whether BFA also affects the trans-most cisternae of the Golgi stack and the trans-Golgi network seems to be dependent on tissue and cell type. Thus, in pancreatic acinar cells, exit from the Golgi complex and formation of secretory granules are not BFA-sensitive (6), while in other cells the formation of secretory granules is inhibited (7). Secretion of preformed secretory granules is not inhibited by BFA. Thus, the effects of BFA point to a common mechanism in the traffic of proteins that follow either the constitutive or the regulated pathways of exocytosis.
BFA's effects are not limited to the Golgi apparatus and are reiterated throughout the endosome/lysosome system. BFA treatment induces tubulation of these compartments (8). Similar to the mixing of the Golgi with the ER, the trans-Golgi network mixes with the recycling endosomal system. Remarkably, this mixed system remains partly functional, with normal cycling between plasma membrane and endosomes, but with impaired traffic between endosomes and lysosomes (8).
This suggests that the vesicle-budding mechanisms in the endocytic pathway are similar to, yet distinct from, those in the exocytic pathway. Furthermore, these observations reinforce the plasticity of the endocytic pathway, which is largely functional even when some traffic steps are inhibited.
The major effects of BFA on protein traffic are explained by its ability to prevent binding of cytosolic coat proteins onto membranes (9). The target of BFA's action is a nucleotide exchange factor for ADP-ribosylation factor (ARF), a small GTP-binding protein. BFA inhibits the ability of Golgi membranes to catalyze the exchange of GTP onto ARF specifically, thereby preventing ARF from interacting with the membrane. As a result, the association of the coat protein b-COP with the Golgi membrane is inhibited (10, 11). b-COP is a subunit of a cytosolic protein complex, the coatomer, that reversibly associates with Golgi membranes and controls vesicular transport.
One possible mode of ARF action is via its ability to stimulate phospholipase D in Golgi membranes (12). Phospholipase D converts phospholipids into phosphatidic acid and thus can alter the lipid content of membranes. Stimulation of the Golgi-associated phospholipase D activity is BFA-sensitive, suggesting a possible link between transport events and the underlying architecture of the lipid bilayer.
The pathogenic bacterium Staphylococus aureus mimics the action of BFA and disassembles the Golgi apparatus (13, 14). This effect is mediated by the secretion of EDIN (epidermal-cell differentiation inhibitor), an extracellular enzyme that ADP-ribosylates Rho GTPase. As a result, all the manifestations of BFA treatment are reproduced. Thus, the regulatory circuit of coat-membrane assembly may involve more than one small GTP-binding protein. In addition to the effects of the small GTP-binding proteins, heterotrimeric G proteins of the Gi/Go subfamily also contribute to the regulation of the cycle of coatomer binding (15, 16). Activation of heterotrimeric G proteins promotes binding of b-COP to Golgi membranes and antagonizes the effect of BFA.
Several BFA-resistant cell lines exist, including the PtK1 rat kangaroo cell line, a derivative of monkey kidney Vero cells, and two derivatives of the human epidermoid carcinoma KB cell line (17). The BFA resistance is dominant and is due to a Golgi-associated factor that is homologous to the target of BFA in cells that are sensitive to the drug. In Golgi membranes from BFA-resistant PtK1 cells, the basal phospholipase D activity is high and insensitive to BFA (12), suggesting a mechanism for the drug resistance. Another mechanism may involve a member of the ATP-binding cassette superfamily of transport proteins, as suggested by BFA-resistant mutants in yeast (18).
The ability of BFA to affect budding of clathrin-coated vesicles from the trans-Golgi network ) observed in many, but not all cells) is mediated through the rapid and reversible redistribution of g-adaptin (19). This component of the clathrin coat is specific to Golgi-derived coated vesicles and is absent from plasma membrane-derived coated vesicles. The kinetic and pharmacological similarities between BFA's effects on g- adaptin and b-COP underscore the biochemical similarities between membrane budding mechanisms that are mediated by various coat complexes.
The effects of BFA are readily reversible, and this reversibility is due partly to its detoxification. The mechanism of BFA detoxification was studied in CHO cells and is mediated by the glutathione S-transferase system via conversion of the antibiotic to its glutathionyl and cysteinyl derivatives, followed by secretion (20).
BFA affects cellular compartmentalization not only via protein traffic, but also through lipid metabolism (21). Its main effect on lipids is enhanced hydrolysis of sphingomyelin, a key regulator of cell proliferation and differentiation. Sphingomyelin is produced from ER-derived ceramide and is delivered to other membranes, presumably via the same trafficking system used for proteins. The effect of BFA on the level of sphingomyelin seems to be a result of the mixing of cellular membranes. BFA treatment also increases the rate of sphingomyelin biosynthesis from phosphatidylcholine, and this effect may be related to the above-mentioned action on phospholipase D. In this context, it is noteworthy that C6 ceramide, a cell-permeable ceramide analogue, partially restores BFA sensitivity in a BFA-resistant cells (22).
The various effects of BFA on a number of cellular membranous organelles indicate both the common and distinct mechanisms that operate to sort membrane components. BFA seems to inhibit a common key step in the mechanism of vesicle budding, namely, regulation of the nucleotide status of different small G proteins. The BFA-sensitive G proteins are each endowed with organelle specificity, coupled perhaps to tissue specificity, which explains the variable sensitivity of membranes to BFA. On the other hand, the use of BFA has also revealed a physiological connection between cellular signal transduction pathways and the traffic of membrane lipids and proteins through the cell.
References
1.Y. Misumi, Y. Misumi, K. Miki, A. Takatsuki, G. Tamura, and Y. Ikehara (1986) J. Biol. Chem., 261, 11398–11403.
2.J. Lippincott-Schwartz, L. C. Yuan, J. S. Bonifacino, and R. D. Klausner (1989) Cell 56, 801–813.
3.J. Lippincott-Schwartz, J. G. Donaldson, A. Schweizer, E. G. Berger, H. P. Hauri, L. C. Yuan, and R. D. Klausner (1990) Cell 60, 821–836.
4.N. E. Ivessa, C. De Lemos-Chiarandini, Y. S. Tsao, A. Takatsuki, M. Adesnik, D. D. Sabatini, and G. Kreibich (1992) J. Cell Biol. 117, 949–958.
5.R. D. Klausner, J. G. Donaldson, and J. Lippincott-Schwartz (1992) Cell Biol. 116, 1071–1080.
6.L. C. Hendricks, S. L. McClanahan, G. E. Palade, and M. G. Farquhar (1992) Proc. Nat. Acad. Sci. USA 89, 7242–7246.
7. S. G. Miller, L. Carnell, and H. H. Moore (1992) J. Cell Biol. 118, 267–283.
8.J. Lippincott-Schwartz, L. Yuan, C. Tipper, M. Amherdt, L. Orci, and R. D. Klausner (1991)Cell 67, 601–616.
9. L. Orci, M. Tagaya, M. Amherdt, A. Perrelet, J. G. Donaldson, J. Lippincott-Schwartz, R. D. Klausner, and J. E. Rothman (1991) Cell 64, 1183–1195.
10. J. G. Donaldson, J. Lippincott-Schwartz, G. S. Bloom, T. E. Kreis, and R. D. Klausner (1990) J. Cell Biol. 111, 2295–2306.
11. J. G. Donaldson, D. Finazzi, and R. D. Klausner (1992) Nature 360, 350–352.
12. N. T. Ktistakis, H. A. Brown, P. C. Sternweis, and M. G. Roth (1995) Proc. Nat. Acad. Sci. USA 92, 4952–4956.
13. M. Sugai, C. H. Chen, and H. C. Wu (1992) J. Biol. Chem. 267, 21297–21299.
14. M. Sugai, C. H. Chen, and H. C. Wu (1992) Proc. Nat. Acad. Sci. USA 89, 8903–8907.
15. J. G. Donaldson, R. A. Kahn, J. Lippincott-Schwartz, and R. D. Klausner (1991) Science 254, 1197-1199.
16.N. T. Ktistakis, M. E. Linder, and M. G. Roth (1992) Nature 356, 344–6.
17.N. T. Ktistakis, M. G. Roth, and G. S. Bloom (1991) J. Cell Biol. 113, 1009–1023.
18.T. G. Turi and J. K. Rose (1995) Biochem. Biophys. Res. Commun. 213, 410–418.
19.M. S. Robinson and T. E. Kreis (1992) Cell 69, 129–138.
20. A. Bruning, T. Ishikawa, R. E. Kneusel, U. Matern, F. Lottspeich, and F. T. Wieland (1992) J. Biol. Chem. 267, 7726–32.
21. C. M. Linardic, S. Jayadev, and Y. A. Hannun (1992) J. Biol. Chem. 267, 14909–14911.
22. T. Oda, C. H. Chen, and H. C. Wu (1995) J. Biol. Chem. 270, 4088–4092.



|
|
|
|
إجراء أول اختبار لدواء "ثوري" يتصدى لعدة أنواع من السرطان
|
|
|
|
|
|
|
دراسة تكشف "سببا غريبا" يعيق نمو الطيور
|
|
|
|
|
|
لأعضاء مدوّنة الكفيل السيد الصافي يؤكّد على تفعيل القصة والرواية المجسّدة للمبادئ الإسلامية والموجدة لحلول المشاكل المجتمعية
|
|
|
|
قسم الشؤون الفكرية يناقش سبل تعزيز التعاون المشترك مع المؤسّسات الأكاديمية في نيجيريا
|
|
|
|
ضمن برنامج عُرفاء المنصّة قسم التطوير يقيم ورشة في (فنّ الٕالقاء) لمنتسبي العتبة العباسية
|
|
|
|
وفد نيجيري يُشيد بمشروع المجمع العلمي لحفظ القرآن الكريم
|