


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-1-2020
Date: 19-9-2020
Date: 23-5-2017
|
The hydroboration reaction is among the few simple addition reactions that proceed cleanly in a syn fashion. As noted above, this is a single-step reaction. Since the bonding of the double bond carbons to boron and hydrogen is concerted, it follows that the geometry of this addition must be syn. Furthermore, rearrangements are unlikely inasmuch as a discrete carbocation intermediate is never formed. These features are illustrated for the hydroboration of α-pinene.
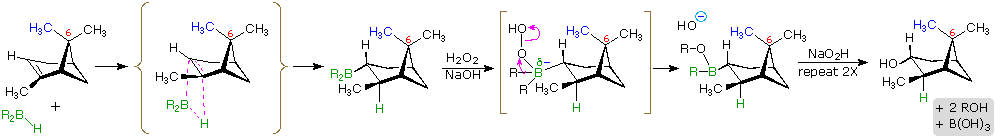
Since the hydroboration procedure is most commonly used to hydrate alkenes in an anti-Markovnikov fashion, we also need to know the stereoselectivity of the second oxidation reaction, which substitutes a hydroxyl group for the boron atom. Independent study has shown this reaction takes place with retention of configuration so the overall addition of water is also syn.
The hydroboration of α-pinene also provides a nice example of steric hindrance control in a chemical reaction. In the less complex alkenes used in earlier examples the plane of the double bond was often a plane of symmetry, and addition reagents could approach with equal ease from either side. In this case, one of the methyl groups bonded to C-6 (colored blue in the equation) covers one face of the double bond, blocking any approach from that side. All reagents that add to this double bond must therefore approach from the side opposite this methyl.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ضمن أسبوع الإرشاد النفسي.. جامعة العميد تُقيم أنشطةً ثقافية وتطويرية لطلبتها
|
|
|