


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-11-2015
Date: 29-10-2015
Date: 18-11-2015
|
Rhabdoviruses
Among the rhabdoviruses, the lyssaviruses, genotypes 1-7, are human pathogens. They are transmitted by the bite of an infected animal in its saliva and infections, once fully manifest, are always lethal (rabies, hydrophobia). The reservoir for type 1 is provided by wild animals in general (foxes, etc.), bats (sylvatic rabies), and, in Asia, dogs (urban rabies). Types 2-7 are restricted to Europe, Asia, Africa, and Australia with their main reservoir in bats.
Diagnosis: direct detection with IF in cornea cells and skin biopsies, postmortem isolation from brain tissues.
Prevention: due to the week-long and even month-long incubation period (except in types 2-4), postexposure prophylactic vaccination with combined active (dead vaccine) and passive (human immunoglobulin) vaccines is possible. Pre-exposure prophylaxis in the form of dead vaccine is administered to persons at high risk.
Pathogen. The rhabdoviruses of significance in human medicine are classified in seven genotypes. Type 1 is the classic, worldwide type that occurs in two forms: the “street virus” isolated from humans and animals and the “virus fixe” according to Pasteur. In 1882, Pasteur had transmitted the virus intracerebrally to rabbits. Following repeated passages of the virus in the rabbits, he had developed a dead vaccine type. Due to the brain-to-brain passages in the laboratory animals, the “virus fixe” became so highly adapted to brain tissue that it was unable to replicate in extraneural tissues. Types 2-4 were isolated from African bats, types 5 and 6 from European bats, and type 7 from Australian bats.
Rhabdoviruses are rodlike, 60 x 180 nm in size, with one end flat and one end rounded (“bulletshaped”) and a spiked envelope surrounding a nucleocapsid similar to that of the myxoviruses. The genome consists of antisense- strand RNA.
Pathogenesis and clinical picture. Rabies viruses are almost always transmitted by the bite, sometimes also the scratch, of a rabid animal (exceptions, see below). The virus at first replicates at the portal of entry in muscle and connective tissue, then wanders along the nerve cells into the CNS, where more viral replication takes place. Using the same route, the virus then disseminates from the CNS into peripheral organs, above all the salivary glands, cornea, and kidneys. The primary clinical picture is an encephalitis with lethal outcome for humans and animals once it has broken out.
Diagnosis. An intravitam laboratory diagnosis is established by examining an impression preparation from the cornea or skin biopsies with immunofluorescence. Postmortem, rabies viruses can be found in the brain tissue of humans and animals by inoculating newborn mice or cell cultures with brain tissue or saliva.
Because antibody production begins so late, serodiagnosis is not practicable. Serological analysis is used to check for vaccine protection. Useful technical tools include an EIA or neutralization test (RFFIT, rapid fluorescent focus inhibition test in cell cultures). Special laboratories are used for the diagnostic testing.
Epidemiology. Lyssavirus type 1 is endemic to North America and Europe in wild animals (sylvatic rabies) and in certain tropical areas in domestic pets as well, in particular dogs (urban rabies). The reservoir for the remaining lyssa- virus types are bloodsucking (hemovorous) as well as fructivorous and insectivorous bats.
The virus is excreted with the saliva of the diseased or terminal incubator animal and enters other animals or humans through scratch or bite wounds.
The virus is highly labile, so transmission on contaminated objects is very rare. Human-to-human transmission has not been confirmed with the exception of cases in which rabies in corneal donors had gone unnoticed.
Prevention. The long incubation period of the rabies virus—in humans several weeks to several months, depending on the localization and severity of the bite wound—makes postexposure protective vaccination feasible. Development of the vaccine originated with Pasteur, who used a dead vaccine from the neural tissues of infected animals. Use of this original rabies vaccine often resulted in severe side effects with allergic encephalomyelitis. The vaccine types in use today are produced in diploid human embryonal cells (HDCV = human diploid cell vaccine), hen fibroblasts or duck embryos. No further adverse reactions have been described with these vaccines, so that 8 earlier apprehensions about the rabies vaccine are no longer justified.
The postexposure procedure depends on the type of contact, the species and condition of the biting animal and the epidemiological situation (Table 1). Exposure is constituted by a bite, wound contamination with saliva or licking of the mucosa, but not by simple petting. In endemic regions, any animal that bites unprovoked must be suspected of being rabid.
Postexposure prophylaxis begins with a rigorous wound toilet, the most important part of which is thorough washing out of the wound with soap, water, and a disinfectant agent. Passive immunization with 20 lU/kg human rabies immunoglobulin (RIG) is then begun, whereby half of the dose is instilled around the wound and the other half is injected i.m. Concurrently, active immunization is started with six doses of HDVC injected i.m. on days 0,3,7,14,30, and 90. The current therapeutic measures are summarized in Table 3.
Important: postexposure vaccination is apparently ineffective against the African viral strains (types 2-4).
Table 1 Rabies: Postexposure Prophylaxis (according to WHO recommendations issued in Geneva, 1992)
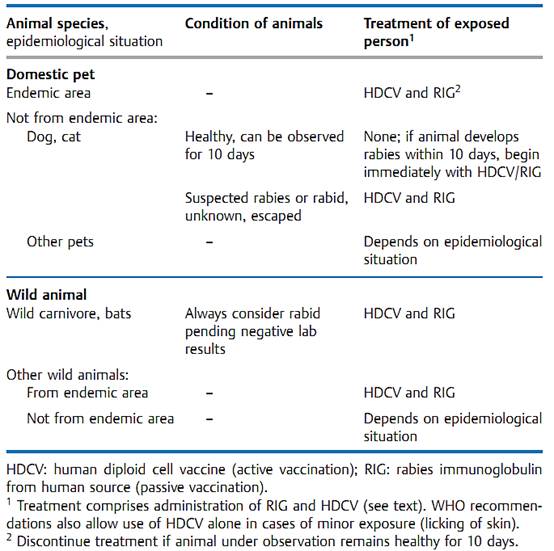
Persons exposed to an increased risk of contracting rabies can also be given pre-exposure protection with three doses of HDCV. Postexposure treatment is then limited to the wound toilet and HDCV injections.
Postexposure prophylaxis is impracticable in animals. Dogs and cats in particular must be vaccinated with living vaccine grown in duck embryos. In wild animals (foxes), oral bait vaccination programs have been successful. If the bait contains the attenuated rabies virus, exposure to it must be considered rabies exposure and the postexposure prophylactic procedure must be carried out. This does not apply to use of the recombinant vaccinia virus. However, on the pathogenicity of the vaccinia virus .



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
الأمين العام للعتبة العسكرية المقدسة يستقبل شيوخ ووجهاء عشيرة البو بدري في مدينة سامراء
|
|
|