


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 20-11-2015
Date: 18-11-2015
Date: 18-11-2015
|
Defense Mechanisms
The mechanisms available to the human organism for defense against viral infection can be classified in two groups. The nonspecific immune defenses, in which interferons play a very important part, come first. Besides their effects on cell growth, immune response, and immunoregulation, these substances can build up a temporary resistance to a viral infection. Interferons do not affect viruses directly, but rather induce cellular resistance mechanisms (synthesis of “antiviral proteins”) that interfere with specific steps in viral replication. The specific immune defenses include the humoral immune system, consisting mainly of antibodies, and the cellular immune system, represented mainly by the T lymphocytes. In most cases, cellular immunity is more important than humoral immunity. The cellular system is capable of recognizing and destroying virus-infected cells on the surfaces of which viral antigens are expressed. The humoral system can eliminate only extracellular viruses.
Nonspecific Immune Defenses
The nonspecific immune defense mechanisms are activated immediately when pathogens penetrate the body's outer barriers. One of the most important processes in these basic defenses is phagocytosis, i.e., ingestion and destruction of pathogens. Granulocytes and natural killer cells bear most of the responsibility in these mechanisms. Changes in pH and ion balance as well as fever also play a role, for example, certain temperature-sensitive replication steps can be blocked. The most important humoral factor is the complement system. Interferons, which are described below, are also potent tools for fighting off viral infections.
Interferons (IFN) are cell-coded proteins with a molecular weight of about 20 kDa. Three types are differentiated (leukocyte interferon = IFNa, fibroblast interferon = IFNb, and immune interferon = IFNy) of which the amino acid sequences are known and which, thanks to genetic engineering, can now be produced in practically unlimited amounts. Whereas the principal biological effects of interferons on both normal and malignant cells are antiviral and antimitotic, these substances also show immunomodulatory effects. Their clinical applications are designed accordingly. In keeping with the scope of this section, the following description of their antiviral activity will be restricted to the salient virological aspects (Fig. 1):
A number of substances can induce the production of interferon in a cell, for example double-stranded RNA, synthetic or natural polynucleotides, bacteria, various low-molecular compounds and, above all, viruses. All of these substances have the same effect: they derepress the cellular interferon gene, inducing the cell to begin producing interferon precursors. Following glyco- sylation, the finished interferon is released into the surrounding area and binds to the interferon receptor of the nearest cell. The presence or lack of this receptor determines what effect the interferon will have. It also explains the more or less pronouncedly species-specific nature of the cell-to-interfer- on relationship. In principle, the effect of interferon is strongest within the species in which it was produced. Within the recipient or “target” cell the interferon induces the expression of the so-called interferon-stimulated genes (ISG) by means of a signal cascade, the result of which is to inhibit viral replication.
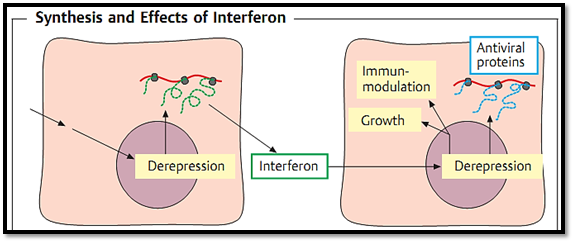
Fig. 1 An interferon-inducing substance initiates production of interferon in the first cell. In the second cell, interferon induces, for instance, production of antiviral proteins.
Mx protein. The observation that certain mice are resistant to influenza viruses led to the discovery of the interferon-induced, 75-80 kDa Mx proteins coded for by dominant hereditary Mx genes. Mx proteins accumulate in mouse cell nuclei and inhibit the mRNA synthesis of influenza viruses. Mx- mice are killed by influenza. In humans, Mx proteins accumulate in the cytoplasm, but their mechanism of action is unknown.
Specific Immune Defenses
The specific, adaptive immune defenses include both the humoral system (antibody-producing B cells) and the cellular system (T helper cells and cytotoxic T lymphocytes). In general, viruses the antigens of which are expressed on the surface of the infected cells tend to induce a cellular immune response and viruses that do not change the antigenicity of their host cells tend to activate the humoral system.
Humoral immunity. Antibodies can only attack viruses outside of their host cells, which means that once an infection is established within an organ it can hardly be further influenced by antibodies, since the viruses spread directly from cell to cell. In principle, the humoral immune system is thus only capable of preventing a generalized infection, but only if the antibodies are present at an early stage (e.g., induced by a vaccination). Class IgG and IgM antibodies are active in the bloodstream (see Chapter 2) and class IgA is active on the mucosal surface. The effect of the antibodies on the viral particles (“neutralization”) is based on steric hindrance of virus adsorption to the host cells by the antibodies attached to their surfaces. The neutralizing effect of antibodies is strongest when they react with the receptor-binding sites on the capsids so as to block them, rendering the virus incapable of combining with the cellular receptors.
Cellular immunity. This type of immune defense is far more important when it comes to fighting viral infections. T lymphocytes (killer cells) recognize virus-infected cells by the viral antigens on their surfaces and destroy them. The observation that patients with defective humoral immunity generally fare better with virus infections than those with a defective cellular response underlines the fact that the cellular immune defense system is the more important of the two.
References
Fritz H. Kayser, M.D. Emeritus Professor of Medical Microbiology Institute of Medical Microbiology, University of Zurich, Zurich, SwitzerlandThieme 2005, Stuttgart ! New York.



|
|
|
|
مخاطر عدم علاج ارتفاع ضغط الدم
|
|
|
|
|
|
|
اختراق جديد في علاج سرطان البروستات العدواني
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|