


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 19-9-2018
التاريخ: 17-10-2020
التاريخ: 3-11-2019
التاريخ: 23-7-2019
|
By now you should be familiar with position isomers wherein compounds of the same molecular formula differ because substituents, chain branches, and so on, are not at the same positions in the molecules. 1-Chloropropane and 2-chloropropane are straightforward examples of position isomers. A much more subtle form of isomerism is present when two different compounds have the same molecular formulas, the same substituent and chain-branching positions, and, indeed, even have the same names by all of the nomenclature rules we have given you so far. Such isomers are different because their molecules have different arrangements of the atoms in space. These are stereoisomers and this type of isomerism, called stereoisomerism, is of enormous importance to all areas of organic chemistry and biochemistry.
To understand stereoisomerism of carbon compounds, we must understand the ways in which the bonds to carbon atoms are arranged in space. this depends on whether the carbon atoms form single, double, or triple bonds to another atom. Thus, four single bonds to a carbon form a tetrahedral arrangement; two single bonds and one double bond to a carbon give a planar array with bond angles near 120o, while one single bond and one triple bond (or two double bonds) to a carbon are arranged linearly:
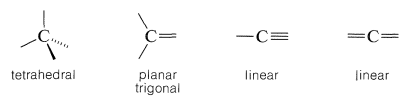



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|