Pelvic Viscera
Pelvic viscera represent portions of the urinary, reproductive, and digestive systems. Pelvic viscera are protected and supported by the bony limits of the pelvis and the muscular and fascial confines of the pelvic walls and floor. The order of structures from anterior to posterior in the female is bladder, uterus/vagina, and rectum (Fig. 1). For males, it is bladder/prostate and rectum (Fig. 2). Important relationships exist between pelvic vasculature and viscera. Development of each of these systems will be detailed, followed by gross anatomy and histology.
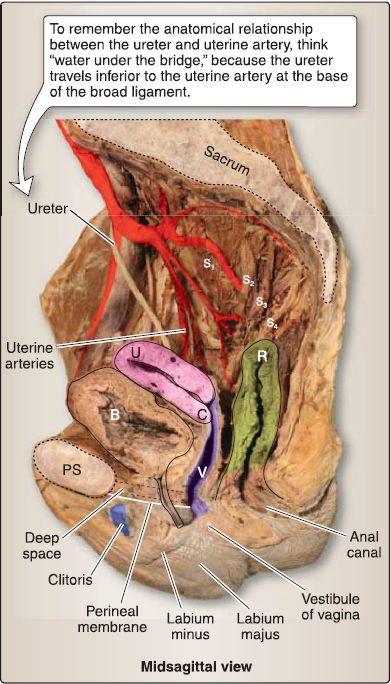
Figure 1 : Female pelvic viscera. B = bladder, C = cervix, PS = pubic symphysis, R = rectum, U = uterus, V = vagina.
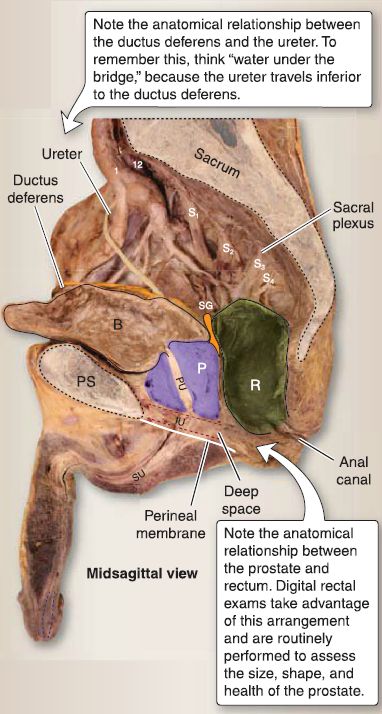
Figure 2 : Male pelvic viscera. B = bladder, IU = intermediate urethra, P = prostate
gland, PS = pubic symphysis, PU = prostatic urethra, R = rectum, SG = seminal gland, SU = spongy urethra.
A. Urinary system
The urinary system consists of the kidneys, ureters, urinary bladder, and urethra. Although the kidneys and proximal ureters are located in the abdomen, they will be included in this section, as will the suprarenal gland, for completeness.
1. Kidney and ureter development: The intermediate mesoderm forms a longitudinal elevation along the dorsal body wall called the urogenital ridge. A portion of the urogenital ridge forms the nephrogenic cord, which gives rise to the urinary system. The nephrogenic cord develops into the pronephros, the mesonephros, and the metanephros (Fig. 3).

Figure 3 : Kidney embryology. A, Early embryology. B, Week 6, week 8, week 12, adult C, Table of Adult Derivatives.
a. Pronephros: At the start of week 4, the pronephros develops by the differentiation of mesoderm within the nephrogenic cord to form pronephric tubules and the pronephric duct. The pronephros is the cranialmost nephric structure and is a transitory structure that regresses completely by week 5. The pronephros is not functional in humans.
b. Mesonephros: At the end of week 4, the mesonephros develops by the differentiation of mesoderm within the nephrogenic cord to form mesonephric tubules and the mesonephric duct (Wolffian duct). The mesonephros is the middle nephric structure and is a partially transitory structure. Most of the mesonephric tubules regress, but the mesonephric duct persists and opens into the urogenital sinus. The mesonephros is functional for a short period.
c. Metanephros: At the start of week 5, the metanephros develops from an outgrowth of the mesonephric duct, the ureteric bud, and from a condensation of mesoderm within the nephrogenic cord, the metanephric mesoderm. The metanephros is the caudalmost nephric structure. The metanephros is functional in the fetus at about week 10. It develops into the definitive adult kidney. The fetal kidney is divided into lobes, in contrast to the definitive adult kidney, which has a smooth contour.
[1] Ureteric bud: The ureteric bud initially penetrates the metanephric mesoderm and then undergoes repeated branching to eventually form the ureter, renal pelvis, major calyx, minor calyx, and collecting duct (CD). At the tip of each branch of the ureteric bud, the metanephric mesoderm aggregates as a cap called cap mesoderm. The cap mesoderm plays an inductive role in the formation of the metanephric vesicles, which later give rise to primitive S-shaped renal tubules.
The S-shaped renal tubules differentiate into the connecting tubule, distal convoluted tubule (DCT), loop of Henle, proximal convoluted tubule (PCT), and the Bowman capsule. The surrounding metanephric mesoderm forms tufts of capillaries called glomeruli that protrude into the Bowman capsule. Nephron formation is complete at birth, but functional maturation of nephrons continues throughout infancy. d. Relative ascent of the kidneys: The fetal metanephros is located at vertebral level S1-S2, whereas the definitive adult kidney is located at vertebral level T12-L3. The change in location results from a disproportionate growth of the embryo caudal to the metanephros. During the relative ascent, the kidney rotates 90°, which causes the hilum, which initially faces ventrally, to finally face medially.
e. Kidney blood supply: During the relative ascent of the kidneys, the kidneys receive their blood supply from arteries at progressively higher levels until the definitive renal arteries develop at L2. Some of the arteries that form during the ascent may persist as end arteries and are called supernumerary arteries. Therefore, any damage to supernumerary arteries (e.g., ligation during surgery) will result in necrosis of kidney parenchyma.
2. Kidney and ureter anatomy: The kidney is a bean-shaped organs located against the posterior abdominal wall bilaterally between T12-L3 vertebral levels . The right kidney is positioned more inferiorly than the left-due to the presence of the liver (Fig. 4). Each kidney (right and left) is surrounded by a perirenal fat capsule and renal fascia. A fibrous capsule lies deep to the perirenal fat. Renal vessels exit and enter the organ at the concave renal hilum, which is found within the renal sinus-a fat-filled space that also houses the renal pelvic and calices. In general, the kidney is comprised of an outer cortex, an inner medulla, and a collecting system.
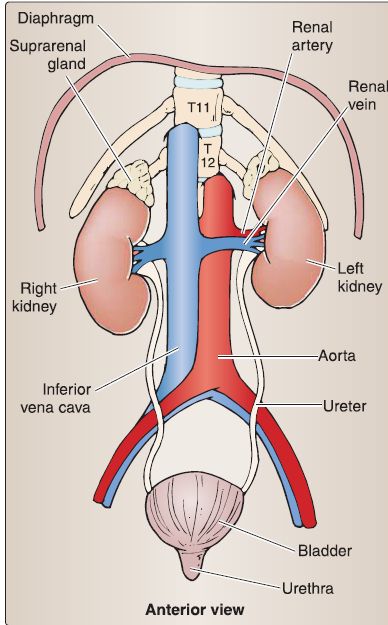
Figure 4 : Urinary system.
a. Cortex and medulla: A coronal slice through the kidney would reveal two-toned parenchyma-the lighter cortex and darker medulla (Fig. 5). Renal pyramids and columns alternate within the kidney substance. The apex of each pyramid is called a renal papilla.
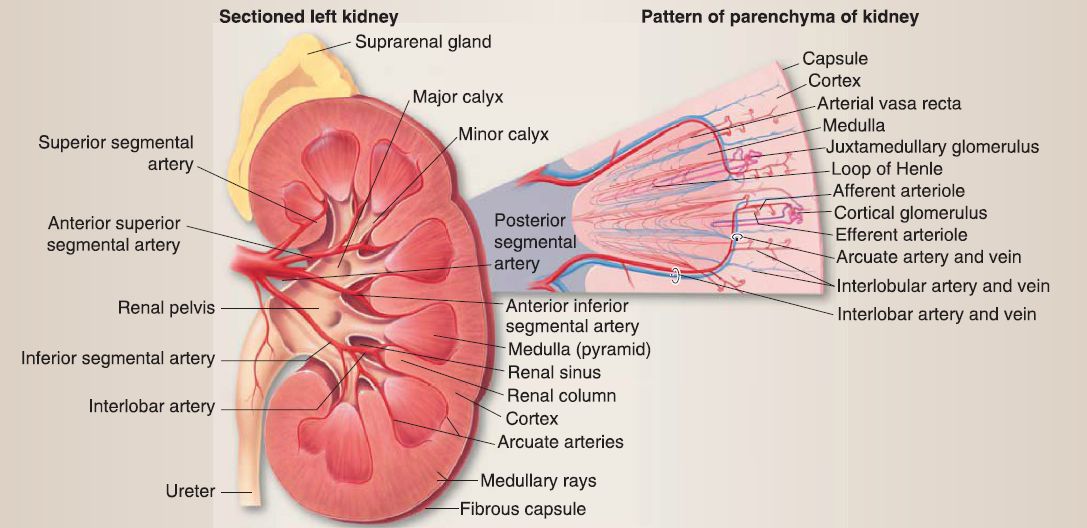 Figure 5 : Kidney gross and microanatomy.
Figure 5 : Kidney gross and microanatomy.
b. Collecting system: At the gross anatomical level, the collecting system consists of minor calyces, major calyces, the renal pelvis, and the ureter. The ureter exits the hilum of the kidney posteriorly to the renal vessels and travels into the pelvis to terminate in the posterior wall of the bladder.
c. Vasculature: Renal arteries and veins supply the kidney. Renal veins drain into the inferior vena cava and are positioned anteriorly to renal arteries, which are branches of the abdominal aorta. The arteries to the ureters are typically branches of renal arteries, gonadal arteries, and the abdominal aorta.
d. Innervation: Kidneys receive autonomic innervation through the renal nerve plexus associated with the least splanchnic and vagus nerves . Along their course in the abdomen and pelvis, the ureters receive fibers from the renal, intermesenteric, superior hypogastric, and inferior hypogastric plexuses.
e. Lymphatics: Lymph from the kidneys follows the renal veins and drains into lumbar lymph nodes . The ureter drains into nodes along its course, including lumbar and iliac (common, external, internal) nodes.
3. Urinary system histology: The kidney comprises the outer cortex, an inner medulla, and a collecting system (Fig. 6). At a microscopic level, additional structural differences are revealed. The intricate CD system is found throughout and connects the cortex and medulla.
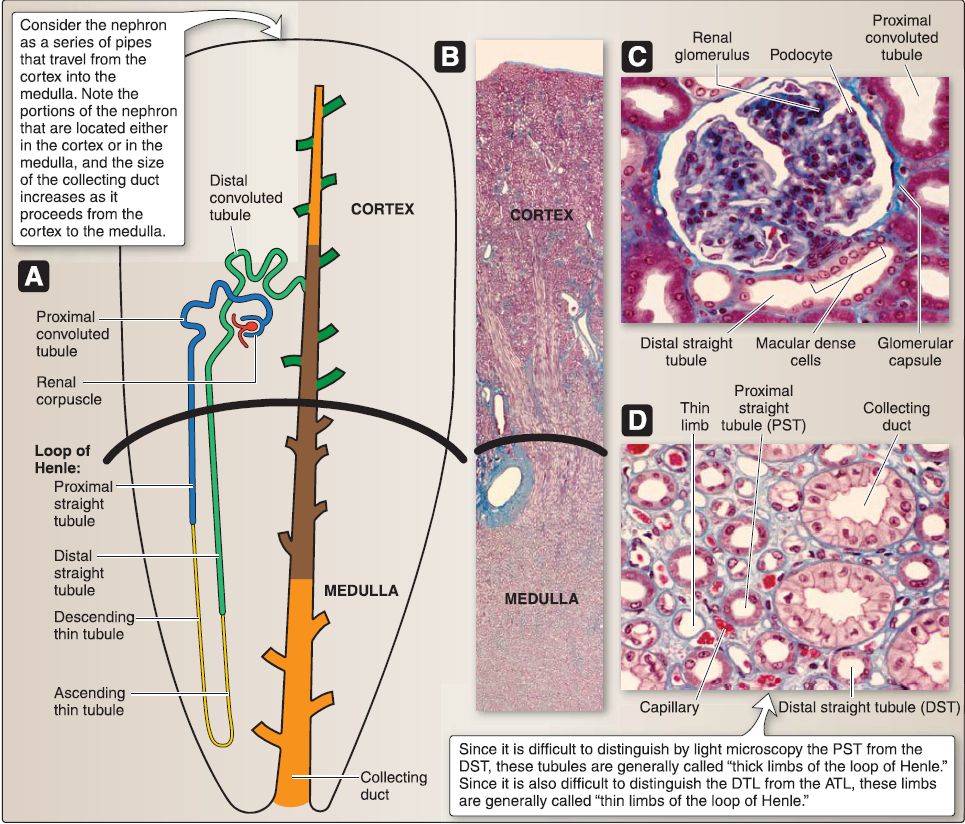 Figure 6: Histology of urinary system. A, Nephron. B, Kidney. C, Cortex. D, Medulla.
Figure 6: Histology of urinary system. A, Nephron. B, Kidney. C, Cortex. D, Medulla.
a. Renal cortex: The renal cortex lies under the connective tissue capsule of the kidney and also extends between the renal pyramids as the renal columns of Bertin. The renal cortex may be divided into the cortical labyrinth and the medullary rays. The cortical labyrinth consists of renal corpuscles (renal glomeruli plus the glomerular [Bowman] capsule), proximal (PCT) and distal convoluted tubules (OCT), interlobular arteries, afferent and efferent arterioles, and a peritubular capillary bed. The medullary rays consist predominately of proximal and distal straight tubules (PST and DST), cortical CDs, and a peritubular capillary bed.
b. Renal medulla: The renal medulla is composed of 5-11 renal pyramids of Malpighi whose tips terminate as 5-11 renal papillae. The base of a renal pyramid abuts the renal cortex, whereas the tip of a renal pyramid (renal papilla) abuts a minor calyx. The renal medulla consists of PSTs, descending and ascending thin limbs (DTL and ATL) of the loop of Henle, DSTs, medullary collecting ducts (CDs), vasa recta, and a peritubular
capillary bed.
c. Collecting system: The collecting system of the kidney includes CDs, minor and major calyces, and the renal pelvis. [1] Collecting ducts: CDs located in the cortex are called cortical collecting ducts. As the cortical CDs travel into the medulla, they are called medullary collecting ducts. As the medullary CDs travel toward the renal papillae, they merge into larger CDs called the papillary ducts of Bellini. The papillary ducts of Bellini open onto the surface of the renal papillae at the area cribrosa. The CDs are lined by a simple cuboidal epithelium that transitions into a simple columnar epithelium as the CDs increase in size toward the renal papillae. The epithelium is composed of two cell types: intercalated (types A and B) and principal.
[2] Minor calyces: The 5-11 minor calyces are cup-shaped structures that abut the 5-11 renal papillae. They consist of a transitional epithelium, a lamina propria rich in collagen and elastic fibers, and a smooth muscle layer.
[3] Major calyces: The 2-3 major calyces are continuous with the minor calyces and have the same composition.
[4] Renal pelvis: The renal pelvis is continuous with the major calyces and has the same composition.
d. Nephron: The nephron is the structural and functional unit of the kidney. It consists of a renal corpuscle, PCT, PST (part of the loop of Henle), DTL and ATL (both part of the loop of Henle), DST (part of the loop of Henle), and the DCT.
[1] Renal corpuscle: A renal corpuscle consists of a renal glomerulus, glomerular (Bowman) capsule, and the intraglomerular mesangium.
(a} Renal glomerulus: This capillary bed (or tuft) consists of a single layer of endothelial cells surrounded by a glomerular basement membrane. The capillaries within the renal glomerulus are continuous, fenestrated capillaries without diaphragms.
(b} Glomerular (Bowman) capsule: This surrounds the renal glomerulus and is double layered. The outer parietal layer consists of a simple squamous epithelium. The inner visceral layer consists of podocytes that extend cell processes to the glomerular basement membrane surrounding the capillaries of the renal glomerulus.
(c} lntraglomerular mesangium: This consists of a mesangial matrix and intraglomerular mesangial cells. The mesangial matrix is an extracellular matrix and contains collagen (types Ill, IV, V, and VI), microfibrillar proteins (fibrillin, MAGP, MP78, MP340), and fibronectin.
[2] Proximal convoluted tubule: The PCT consists of simple cuboidal epithelial cells with a round, centrally located nucleus, a bright eosinophilic (pink) cytoplasm, a distinct apical microvillus border, apical endocytotic vesicles, juxtaluminal zonula occludens (tight junctions), lateral cell processes that interdigitate with adjacent cells, and basal cell processes that associate with numerous mitochondria and contain actin filaments.
[3] Proximal straight tubule: The PST consists of simple cuboidal epithelial cells that are similar in appearance to those of the PCT.
[4] Descending thin limb: The DTL consists of simple squamous epithelial cells with deep juxtaluminal zonula occludens (tight junctions) and a few microvilli. It does not have lateral cell processes.
[5] Ascending thin limb: The ATL consists of simple squamous epithelial cells with shallow juxtaluminal zonula occludens (tight junctions) and extensive lateral cell processes.
[6] Distal straight tubule: The DST consists of simple cuboidal epithelial cells with a round, centrally located nucleus, a pale eosinophilic (pink) cytoplasm, juxtaluminal zonula occludens (tight junctions), lateral cell processes that interdigitate with adjacent cells, and basal cell processes that associate with numerous mitochondria and contain actin filaments. It does not possess an apical microvillus border. The DST contains specialized cells called macula densa (MD) cells in the region of the afferent and efferent arterioles.
[7] Distal convoluted tubule: The DCT is composed like the DST apart from containing MD cells.
e. Collecting ducts: CDs consists of either a simple cuboidal or simple columnar epithelium depending on size. CDs are found in both the renal cortex and renal medulla. They are composed of three cell types: types A and B intercalated and principal cells.
[1] Type A intercalated cell: These are found predominately in cortical CDs and gradually decrease in number in the medullary CDs until they are completely absent in the largest papillary ducts. They have a round and basally located nucleus, distinctive apical microfolds, juxtaluminal zonula occludens (tight junctions), apical endocytotic vesicles, numerous mitochondria, and an W ATPase located on the apical membrane. The type A intercalated cell does not possess lateral cell processes or basal cell processes.
[2] Type B intercalated cell: These are similar in appearance to type A intercalated cells but differ in physiologic function.
[3] Principal cell: These are found in both cortical CDs and medullary CDs. They have a round and basally located nucleus, a few apical microvilli, a single primary cilium, juxtaluminal zonula occludens (tight junctions), basal processes that associate with numerous mitochondria, and AQ2 aquaporin water channels that are regulated by antidiuretic hormone. Principal cells do not have lateral cell processes that interdigitate with adjacent cells.
f. Glomerular filtration barrier: Urine formation begins with filtration, which occurs where the renal glomerulus and the glomerular (Bowman) capsule interact to form the glomerular filtration barrier (GFB). Filtration is the bulk flow of fluid from the glomerular capillaries into the urinary (Bowman) space to form tubular fluid. The GFB comprises the glomerular capillary endothelium, glomerular basement membrane, and slit diaphragms (Fig. 7).

Figure 7: Renal corpuscle and related structure histology
[1] Glomerular capillary endothelium: This is a continuous, fenestrated (without diaphragms) endothelium.
[2] Glomerular basement membrane: This 300-350-nm thick basement membrane is synthesized jointly by the glomerular capillary endothelium and podocytes.
[3] Slit diaphragms: These are found between neighboring podocytes (Fig. 8). The podocyte has a voluminous cell body that bulges into the urinary (Bowman) space and makes up the visceral layer of the glomerular (Bowman) capsule. It gives rise to primary and secondary foot processes and pedicels, which make contact with the GBM. The pedicels from neighboring podocytes regularly interdigitate with one another, thereby forming elongated 25-nm wide spaces called the filtration slits that are bridged by slit diaphragms.
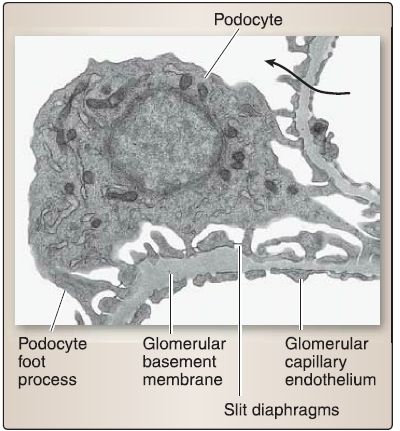
Figure 8: Glomerular filtration barrier histology. Arrow indicates fluid flow from the
capillary lumen to the urinary space.
g. Juxtaglomerular complex: The juxtaglomerular (JG) complex is a specialized structure located where the DST of the nephron makes contact with the vascular pole of the glomerulus (i.e., where the afferent and efferent arterioles are located). The JG complex regulates arterial blood pressure in a slow, long-term, hormonal manner through the renin-angiotensin II mechanism. Its components include MD and JG cells (Fig. 9).
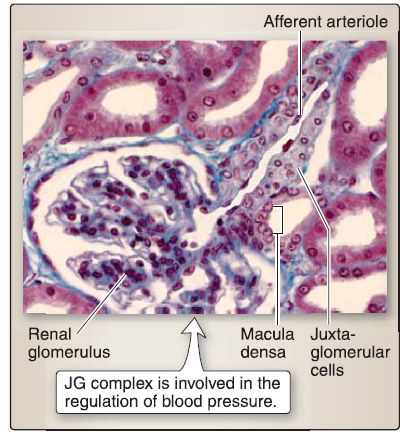
Figure 9 : Juxtaglomerular (JG) complex histology.
[1] Macula densa: This modified epithelial cell lines the DST and is columnar-shaped with a round nucleus. The nuclei of adjacent MD cells are crowded together and may appear to be superimposed on each other. The MD cell monitors changes in Na+ levels in the DST fluid and stimulates the release of renin from JG cells.
[2] Juxtaglomerular cell: This modified smooth muscle cell is located in the tunica media of the afferent arteriole and early portion of the efferent arteriole. It is cuboidal-shaped with a round nucleus and has numerous cell processes, gap junctions that communicate with neighboring cells, and distinct granules that contain renin (a proteolytic enzyme). The JG cell secretes renin, monitors changes in blood pressure, and is innervated by postganglionic sympathetic nerves.
4. Ureter histology: The ureter is organized into a mucosa, smooth muscle layer, and an adventitia (Fig. 10).
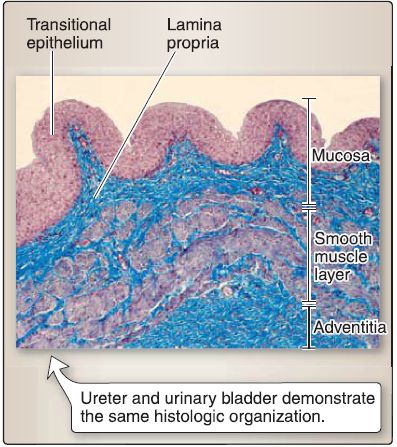
Figure 10: Ureter histology.
a. Mucosa: The mucosa of the ureter consists of a transitional epithelium and a lamina propria. The transitional epithelium is a stratified epithelium, whereby the cells in the surface layer undergo a transition in shape from dome shaped to flat. The lamina propria of the ureter is a dense, irregular connective tissue.
b. Smooth muscle: The smooth muscle layer of the ureter consists of two layers of helically arranged single-unit smooth muscle cells.
c. Adventitia: The adventitia of the ureter consists of dense, irregular connective tissue and retroperitoneal adipose tissue.
5. Urinary bladder development: The urinary bladder is formed from the upper portion of the urogenital sinus, which is continuous with the allantois (Fig. 11). The allantois becomes a fibrous cord called the urachus (or median umbilical ligament in the adult). The lower ends of the mesonephric ducts become incorporated into the posterior wall of the bladder to form the trigone of the bladder. The mesonephric ducts eventually open just below the neck of the bladder. The transitional epithelium lining the urinary bladder is derived from endoderm because of its etiology from the urogenital sinus and gut tube.
 Figure 11 :Urinary bladder development. A, Formation and partitioning by urorectal septum. B, Formation of urogenital sinus and anorectal canal. C, Development of urinary bladder from urogenital sinus.
Figure 11 :Urinary bladder development. A, Formation and partitioning by urorectal septum. B, Formation of urogenital sinus and anorectal canal. C, Development of urinary bladder from urogenital sinus.
6. Urinary bladder and urethra anatomy: The urinary bladder and urethra are continuous and represent the inferior pelvic portion of the urinary system.
a. Urinary bladder: The urinary bladder is a muscular reservoir for urine positioned posterior to the pubic symphysis (Fig. 12). It has an apex, fundus, body, and neck. The detrusor muscle constitutes most of the bladder wall and contributes to the formation of the internal urethral sphincter, which is more prominent in males. Along the internal, posterior surface of the bladder is an inverted triangular region called the trigone. The ureteric orifices mark the two superior corners of the trigone (triangle base), and the apex sits at the internal urethral orifice.

Figure 12 : Urinary bladder and urethra (female).
b. Urethra: In both females and males, the urethra begins at the internal urethral orifice, just inferior to the neck of the bladder.
[1] Female urethra: The female urethra is short and travels posterior and inferior to the pubic symphysis, through the deep perinea! pouch, and ends in the vestibule of the vagina at the external urethral orifice . The posterior wall of the female urethra is anatomically related to the anterior wall of the vagina. Multiple paraurethral glands (also called lesser vestibular glands and Skene glands) are present alongside the urethra and empty through a common duct bilaterally near the external urethral orifice. These glands are thought to aid in lubricating the vestibule.
[2] Male urethra: The male urethra is long and divided into three parts based on adjacent anatomy-prostatic, intermediate (membranous), and spongy parts-which pass through the prostate, deep pouch, and corpus spongiosum, respectively (Fig. 13).
 Figure 13: Male urethra. P = prostate.
Figure 13: Male urethra. P = prostate.
(a) Prostatic: The prostatic urethra has a midline urethral crest, flanked by grooves called prostatic sinuses. Multiple prostatic ducts open into the sinuses. Paired ejaculatory ducts open onto the surface of the urethra crest on either side of the prostatic utricle-a small blind pouch within the mound-shaped seminal colliculus at the peak of the crest. The prostatic urethra receives secretions from the vas deferens (sperm), seminal glands, and prostate to form semen.
(b) Intermediate: The intermediate urethra is the shortest in length of the three portions. It traverses the deep perineal pouch and is flanked by bulbourethral glands, although they do not empty secretions into this portion. The external urethral sphincter surrounds and supports the intermediate urethra and allows for voluntary control of urine flow.
(c) Spongy: The spongy urethra varies in length, but is the longest of the three portions. It enters the bulb of the penis and travels through the corpus spongiosum (body of penis). The proximal end of the spongy urethra receives paired bulbourethral gland ducts. At the distal end, the spongy urethra expands within the glans penis to form the navicular fossa before terminating at the external urethral orifice.
7. Urinary bladder histology: The urinary bladder is organized into a mucosa, smooth muscle layer, and an adventitia (Fig. 14).

Figure 14 : Urinary bladder histology. Inset shows higher magnification of transitional
epithelium, arrow=dome-shaped cell.
a. Mucosa: The mucosa of the urinary bladder consists of a transitional epithelium and a lamina propria. The transitional epithelium is a stratified epithelium, and the cells in the surface layer undergo a transition in shape from dome shaped to flat as the bladder fills with urine. The dome-shaped surface cells demonstrate a characteristic ultrastructural feature called plaques, which are thick, focal areas of the cell membrane associated with actin filaments. The lamina propria of the urinary bladder is a dense, irregular connective tissue.
b. Smooth muscle: The smooth muscle layer of the urinary bladder consists of a complex meshwork of single-unit smooth muscle cells (called the detrusor muscle). In the male, the detrusor muscle forms a complete collar around the neck of the bladder called the internal urethral sphincter and extends longitudinally into the prostatic urethra. In the female, the detrusor muscle does not form a significant sphincter around the neck of the bladder but does extend longitudinally into the urethra.
c. Adventitia: The adventitia of the urinary bladder consists of dense, irregular connective tissue.
8. Suprarenal gland development: The suprarenal gland consists of a cortex and medulla each of which have a separate embryologic origin (Fig. 15).

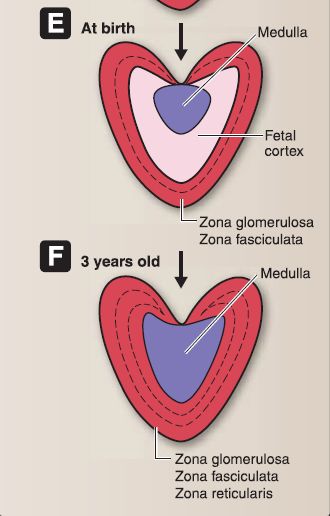
Figure 15 : A-F, Suprarenal gland embryology.
a. Suprarenal cortex: The cortex forms as a result of two episodes of mesoderm proliferation that occur near the root of the dorsal mesentery. During week 5, the first episode begins as mesoderm proliferates, migrates deep into the underlying mesoderm, and forms the inner fetal cortex. During week 6, the second episode begins as mesoderm proliferates, migrates deep into the underlying mesoderm, and forms the outer adult cortex. During the fetal period and at birth, the suprarenal glands are very large due to the size of the fetal cortex. The suprarenal glands become smaller as the fetal
cortex involutes rapidly during the first 2 weeks after birth and continues to involute during the first year of life. The zona glomerulosa (ZG) and zona fasciculata (ZF) of the adult cortex are present at birth, but the zona reticularis (ZR) is not formed until 3 years of age.
b. Suprarenal medulla: The medulla forms when neural crest cells migrate from an area around the neural tube and aggregate at the medial aspect of the fetal cortex. The neural crest cells eventually become surrounded by the fetal and adult cortex. The neural crest cells differentiate into chromaffin cells, which stain yellow-brown with chromium salts.
9. Suprarenal gland anatomy: Although found in the abdominal cavity, the suprarenal {adrenal) glands are detailed here because of their relationship with the kidneys (Fig. 16). Both right and left suprarenal glands sit on the superomedial surface of the right and left kidneys, respectively. The right suprarenal gland is pyramidal, while the left is more crescent shaped. Both glands are encased with the kidney in the perirenal fat capsule, and they are also attached to the respective crura of the diaphragm.
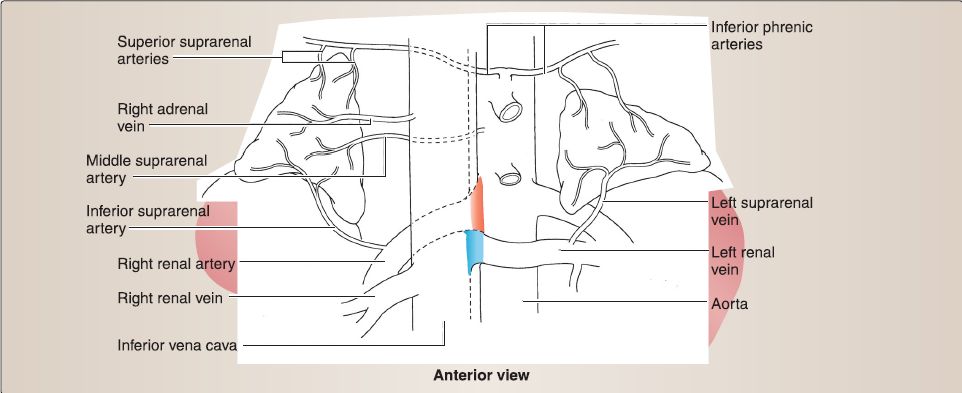 Figure 16 : Suprarenal glands.
Figure 16 : Suprarenal glands.
a. Blood supply: Suprarenal glands are supplied by a series of suprarenal arteries (superior, middle, inferior) and drained by a right or left suprarenal vein. Superior, middle, and inferior suprarenal arteries arise from the inferior phrenic, abdominal aorta, and renal arteries, respectively. Right suprarenal vein drains into the inferior vena cava, while the left drains into the left renal vein.
b. Innervation: Suprarenal glands are unique in that the medulla receives preganglionic sympathetic fibers from the celiac plexus (continuation of greater splanchnic nerve). These axons synapse directly onto chromaffin cells in the suprarenal medulla.
c. Lymphatics: Suprarenal lymph drains into lumbar lymph nodes.
10. Suprarenal gland histology: The suprarenal gland consists of two embryologic distinct components: suprarenal cortex and suprarenal medulla (Fig. 17).
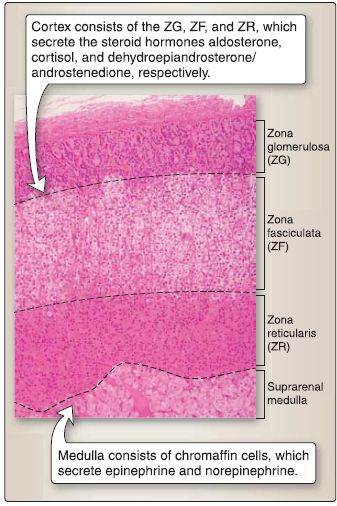
Figure 17: Suprarenal gland histology.
a. Suprarenal cortex: The suprarenal cortex is divided into three zones: ZG, ZF, and ZR.
[1] Zona glomerulosa: The ZG constitutes 15% of cortical volume. ZG cells are arranged in oval-shaped clusters or curved columns that are continuous with the ZF. They are small, pyramidal cells with round, centrally located nucleus. They contain rough endoplasmic reticulum (RER), polyribosomes, prominent smooth endoplasmic reticulum (SER), mitochondria with tubular cristae, and lipid droplets. The ZG cell synthesizes and secretes aldosterone (steroid hormone).
[2] Zona fasciculata: The ZF constitutes 78% of cortical volume. ZF cells are arranged in vertical cords and are large, polyhedral cells with a round, centrally located nucleus. They contain RER, polyribosomes, prominent SER, mitochondria with tubular cristae, numerous conspicuous lipid droplets, and lysosomes. The ZF cell synthesizes and secretes cortisol (steroid hormone).
[3] Zona reticularis: The ZR constitutes 7% of cortical volume. ZR cells are arranged in an anastomosing network of cords. They are small, round cells with a round, centrally located nucleus. They contain RER, polyribosomes, prominent SER, mitochondria with tubular cristae, numerous lipid droplets, and lipofuscin pigment. The ZR cell synthesizes and secretes dehydroepiandrosterone and androstenedione.
b. Suprarenal medulla: The suprarenal medulla contains chromaffin cells, which are modified postganglionic sympathetic neurons. Preganglionic sympathetic axons (via splanchnic nerves) synapse on chromaffin cells and upon stimulation cause chromaffin cells to secrete the catecholamines epinephrine and norepinephrine. Chromaffin cells are derived from neural crest cells and are of two types: epinephrine containing and norepinephrine containing.
[1] Epinephrine-containing cell: These contain RER, polyribosomes, a Golgi complex, mitochondria, and small secretory granules with a low-to-moderate spherical electron-dense core and a minimal electron-lucent halo. The epinephrinecontaining cell secretes epinephrine, and all of the circulating epinephrine in the blood is derived from the suprarenal medulla.
[2] Norepinephrine-containing cell: These contain RER, polyribosomes, a Golgi complex, mitochondria, and large secretory granules with a highly electron-dense spherical core and a conspicuous electron-lucent halo. The norepinephrine-containing cell secretes norepinephrine. The majority of the circulating norepinephrine in the blood is derived from the postganglionic sympathetic neurons and the brain, with the secretion from the suprarenal medulla contributing only a minor portion.
B. Reproductive system
The female and male reproductive systems arise from common embryologic origin but differentiate over the course of fetal development into homologous structures with distinct physiologic functions. The distal portion of the urinary tract-the urethra-is integrated into the male reproductive system as it serves as a conduit for sperm delivery, in addition to urine excretion. In the female, the urethra is functionally separate, but anatomically related to the reproductive system. These relationships explain why the two systems are commonly combined and described as urogenital systems. While urethra anatomy is detailed in the urinary system section, it is also mentioned here briefly for completeness.
1. Reproductive system development: The intermediate mesoderm forms a longitudinal elevation along the dorsal body wall called the urogenital ridge. A portion of the urogenital ridge forms the gonadal ridge, which gives rise to the gonads of the reproductive system. The embryologic formation of the male and female reproductive systems has a common ancestry, which is highlighted by the term indifferent embryo (Fig. 18). In addition, due to the complexity involved in gender determination, the embryo may be considered to have four assignments: genotype, genotype, phenotype, and neurotype. If the genotype, gonotype, phenotype, and neurotype do not align in a congruous manner, a condition of intersexuality exists.
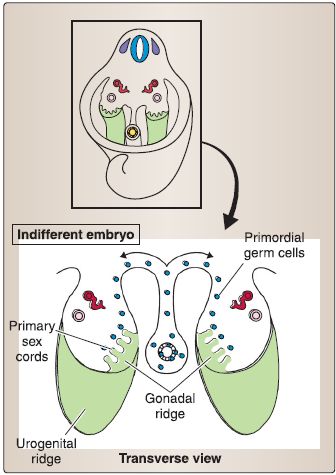
Figure 18: Reproductive system development.
a. Genotype: Genotype depends on whether the chromosome composition is 46, XY (male) or 46, XX (female).
b. Genotype: Genotype depends on whether the indifferent gonad develops into a testes (male) or ovary (female).
c. Phenotype: Phenotype depends on whether the external genitalia (and genital ducts) can be unambiguously identified as male (e.g., penis, scrotum) or female (e.g., clitoris, vagina).
d. Neurotype: Neurotype depends on whether the brain has been sexualized as either masculine or feminine.
e. Indifferent embryo: At fertilization, the genotype of the embryo (46, XY or 46, XX) is established (see Fig. 18). During weeks 1-6, the embryo remains in an indifferent stage called the indifferent embryo. This means that genetically male embryos and genetically female embryos are visibly indistinguishable (e.g., by a sonogram). During week 7, the indifferent embryo begins differentiation along either a male or female pathway. By week 12, male or female characteristics of the external genitalia can be recognized. By week 20, the differentiation of male or female characteristics is complete.
2. Female reproductive system development: The components of the indifferent embryo that are remodeled to form the adult female reproductive system include the gonads (ovaries), genital ducts, and primordia of external genitalia (Fig. 19). The sequence of remodeling begins with the gonads, then the genital ducts, and finally the primordia of the external genitalia, which has already been discussed in the perineum.
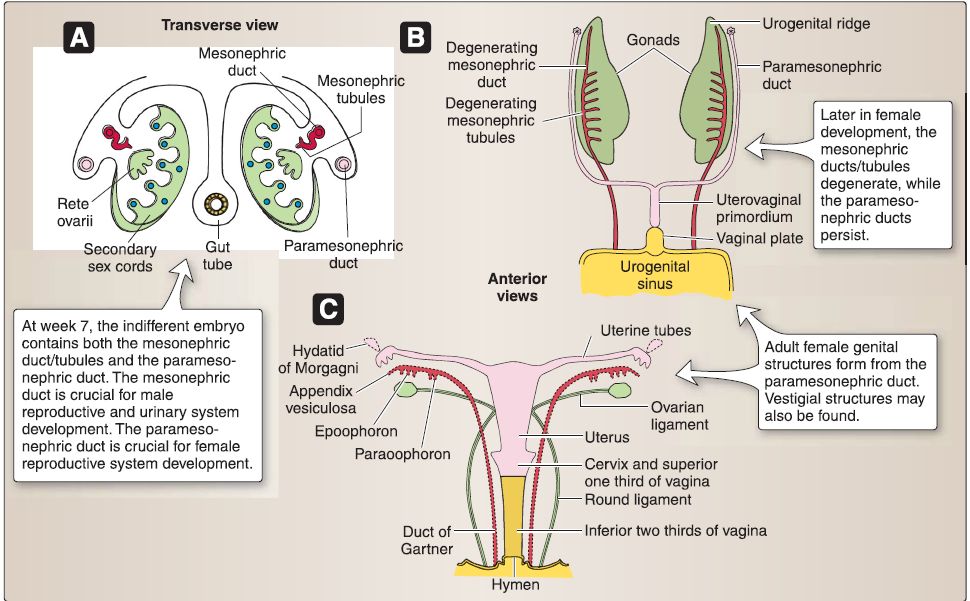 Figure 19 : Female reproductive system embryology. A, Week 12 XX gonad. B, XX gonad and genital duct. C, Primordia of external genitalia.
Figure 19 : Female reproductive system embryology. A, Week 12 XX gonad. B, XX gonad and genital duct. C, Primordia of external genitalia.
a. XX gonad: The coelomic epithelium associated with the gonadal ridge forms somatic support cells within the XX gonad. The somatic support cells do not express the SRY gene located on chromosome Yp11.3, which encodes for the sex-determining
region Y protein (Sry protein) because no Y chromosome is present. In the absence of the Sry protein, somatic support cells within the XX gonad differentiate into follicular cells. In the absence of Sry protein, Mullerian inhibitory factor (MIF), and testosterone, the XX gonad forms the ovary, and the indifferent embryo will be directed toward female characteristics. During week 7, primary sex cords develop from the gonadal ridge and incorporate primordial germ cells (XX genotype), which migrate into the XX gonad from the wall of the yolk sac. The primary sex cords extend into the medulla of the XX gonad and develop into the rete ovarii, which eventually degenerates. Later, secondary sex cords develop and incorporate primordial germ cells as a thin tunica albuginea forms. The secondary sex cords break apart and form isolated cell clusters called primordial follicles. A primordial follicle consists of a primary oocyte surrounded
by a single layer of follicular cells.
b. Female genital ducts: The indifferent embryo-no matter whether it possesses a XY genotype or XX genotype-contains both the mesonephric (Wolffian) ducts/tubules and the paramesonephric (Miillerian) ducts.
[1] Mesonephric: The mesonephric (Wolffian) ducts/tubules in the female degenerate.
[2] Paramesonephric: The cranial portion of the paramesonephric (Mullerian) ducts in the female form the uterine tubes. The caudal portion of the paramesonephric (Mullerian) ducts in the female fuse in the mid line to form the uterovaginal primordium.
The uterovaginal primordium later develops into the uterus, cervix, and superior third of the vagina. The caudal portion of the paramesonephric (Mullerian) ducts in the female projects into the dorsal wall of the cloaca and induces the formation of the sinovaginal bulbs. These fuse to form the solid vaginal plate, which later canalizes and develops into the inferior third of the vagina.
3. Female reproductive system anatomy: The female reproductive system includes the uterine tubes, ovaries, uterus, cervix, and vagina (Fig. 20). These structures are adjacent or continuous with one another and are generally positioned between the bladder and rectum.
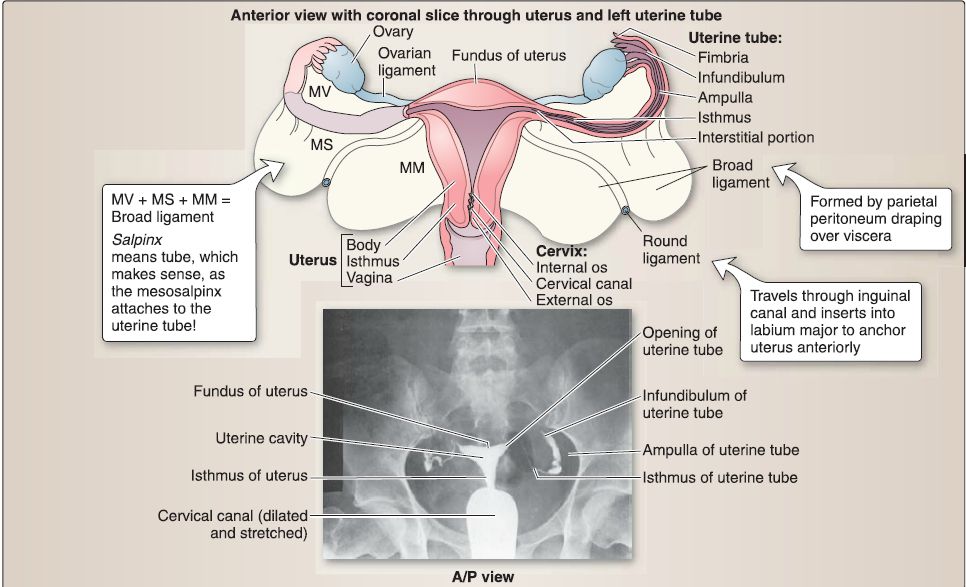 Figure 20 : Female reproductive organs. A, Uterus, ovaries, uterine tubes, cervix, and upper vagina. B, Hysterosalpingograph. A/P = anterior/posterior, MM = mesometrium, MS= mesosalpinx, MV = mesovarium.
Figure 20 : Female reproductive organs. A, Uterus, ovaries, uterine tubes, cervix, and upper vagina. B, Hysterosalpingograph. A/P = anterior/posterior, MM = mesometrium, MS= mesosalpinx, MV = mesovarium.
a. Uterine tubes: The uterine tube is divided into four regions: infundibulum, ampulla, isthmus, and the intramural segment.
[1] lnfundibulum: The infundibulum is the flared open end of the uterine tube next to the ovary. The fimbriae are delicate, finger-like projections that extend from the infundibulum toward the ovary.
[2] Ampulla: The ampulla is the longest segment of the uterine tube and has the largest diameter. This region is where fertilization typically occurs.
[3] Isthmus: The isthmus is the narrow segment of the uterine tube between the ampulla and uterus.
[4] Intramural segment: The intramural segment (1 cm in length) is the portion of the uterine tube contained within the wall of the uterus.
b. Uterus: The uterus is divided into four regions: fundus, cornu,
body, and cervix.
[1] Fundus: The fundus is located superior to the cornu and contributes largely to the upper segment of the uterus during pregnancy. At term, the fundus may extend as high as the xiphoid process (vertebral level T9).
[2] Cornu: The cornu is located near the entry of the uterine tubes.
[3] Body: The body (corpus) is located between the cornu and cervix. The isthmus is part of the body and is the dividing line between the body of the uterus and the cervix.
[4] Cervix: The cervix is located inferior to the body and protrudes into the vagina. The cervix contains the internal os, cervical canal, and external os (Fig. 21). The cervix (2.5-3.0 cm in length) is the lower part of the uterus. The cervix is divided into two portions: the supravaginal portion lies above the vaginal vault, and the vaginal portion protrudes into the vagina. The junction between the cervix and the body of the uterus is at the internal os.
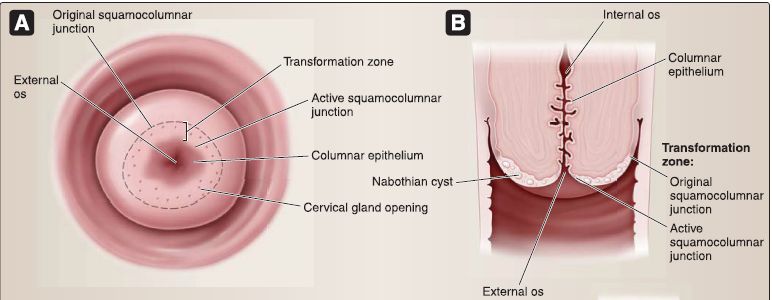

Figure 21 : Cervix.
c. Vagina: The vagina spans between the cervix and vestibule of the vagina and is positioned posterior to the bladder/urethra and anterior to the rectum (Fig. 22). As the vaginal portion of the cervix extends into the superior vagina, a circumferential recess is created. Continuous zones of the recess are called the anterior fornix, posterior fornix, and lateral fornix.
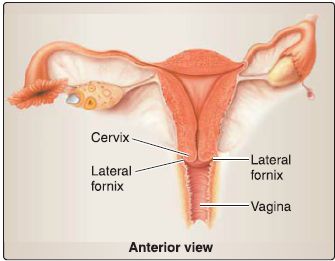
Figure 22 : Vagina.
d. Connective tissue support: In addition to the pelvic floor muscles and endopelvic fascia extensions, the uterus, uterine tubes, and ovaries are supported by a series of connective tissue "ligaments" (Fig. 23).
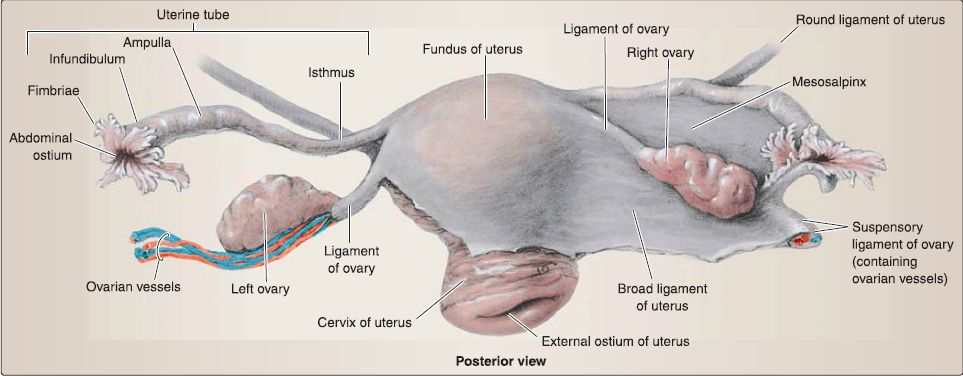 Figure 23 :Female reproductive visceral support.
Figure 23 :Female reproductive visceral support.
[1] Broad ligament: Uterus position is maintained by the broad ligament, which has three parts: mesometrium (attached along uterine body), mesovarium (attached to ovary), and mesosalpinx (attached to uterine tube). Although not a true ligament, this double layer of peritoneum drapes over the uterus, extends along the sides of the uterine body, and attaches to the pelvic walls.
[2] Suspensory ligament of the ovary: Continuous with the broad ligament, the suspensory ligament of the ovary is formed by the double layer of peritoneum draped over the ovarian artery and vein that course into the pelvis from the abdomen.
It supports the position of the ovary and uterine tubes.
[3] Round ligament of the uterus: The round ligament in the female represents the distal end of the gubernaculum, which helped guide the ovary into the pelvis from the abdomen. It extends anterolaterally from the uterus, travels through the inguinal canal, and inserts into the labia majora bilaterally. This ligament helps to maintain the anteverted position of the uterus.
[4] Ligament of the ovary: This ligament represents the proximal end of the gubernaculum and attaches the ovary to the uterus at the uterotubal junction.
e. Vasculature: The uterus is supplied by the uterine arteries, with contributions from branches of the ovarian arteries after supplying the ovaries (Fig. 24). Both of these arteries supply portions of the uterine tubes as well. The superior vagina is supplied by vaginal arteries, while internal pudendal artery branches serve the inferior vagina. Venous drainage occurs by way of the uterine venous plexus, which drains into the uterine veins, tributaries of the iliac veins.
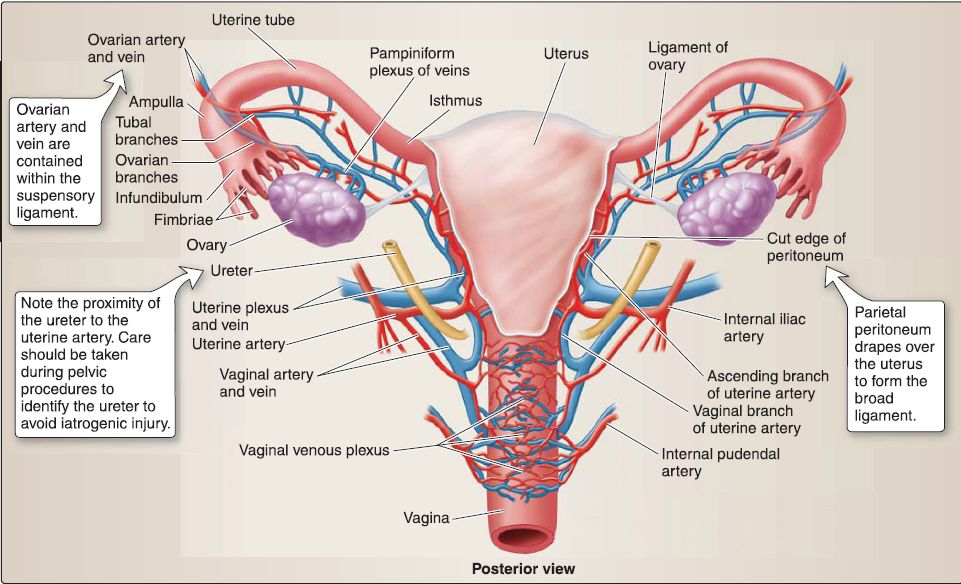 Figure 24 : Vascular supply to female reproductive viscera.
Figure 24 : Vascular supply to female reproductive viscera.
f. Innervation: The uterovaginal nerve plexus, which is a subsidiary of the inferior hypogastric plexus by way of the pelvic plexus, provides autonomic innervation to the uterus and superior vagina . The inferior vagina receives somatic innervation from branches of the pudendal nerve. Visceral sympathetic afferent fibers carrying pain from the uterine body and fundus travel back to inferior thoracic/ superior lumbar ganglia. Visceral afferents from the cervix and vagina follow the parasympathetic motor outflow back to the level of pelvic splanchnics (S2-S4).
g. Lymphatics: Structures of the female reproductive viscera have multiple lymph drainage patterns . Superior structures such as the uterine fund us and superior body may drain into lumbar nodes, while the area adjacent to the round ligament drains through superficial inguinal nodes. Inferior uterine body and cervix drain into external and internal iliac nodes, respectively. Lymphatic drainage of the ovaries follows ovarian lumbar nodes.
4. Female reproductive system histology: This includes the ovary, uterine tube, uterus (menstrual cycle), cervix, and vagina.
a. Ovary: The ovary is an almond-shaped structure located posterior to the broad ligament (Fig. 25). The ovary is covered by a simple cuboidal epithelium called the serosal epithelium with a subjacent connective tissue layer called the tunica albuginea. The ovary is divided into a medulla and cortex.
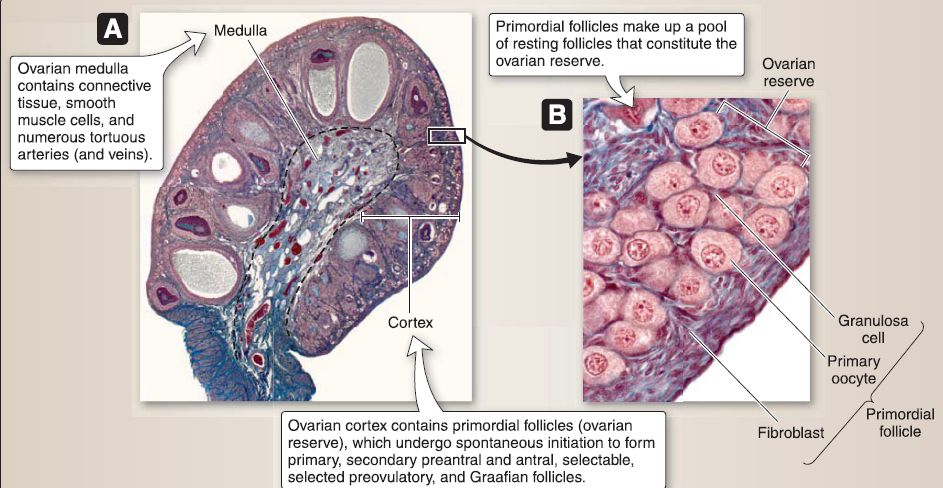

Figure 25: Ovarian histology. A, Ovary. B, Primordial follicle. C, Corpus luteum.
[1] Ovarian medulla: The ovarian medulla lies deep to the cortex and contains connective tissue, occasional smooth muscle cells, and numerous tortuous arteries (and veins) from which small branches radiate to the cortex.
[2] Ovarian cortex: The ovarian cortex contains primordial, primary, secondary preantral, secondary antral, selectable, selected preovulatory, and Graafian follicles. It consists of highly cellular connective tissue stroma and occasional smooth muscle cells.
(a) Primordial follicle (resting stage): Primordial follicle makes up a pool of resting follicles that constitute the ovarian reserve and undergo a spontaneous initiation to form growing follicles. The primordial follicle is characterized by its size (-0.35 mm in diameter) and a single layer of squamous granulosa cells. The primordial follicle consists of three cell types: primary oocyte, granulosa cells, and fibroblasts.
(b) Primary follicle (first stage): A large primary follicle is the first stage in ovarian follicle growth. The large primary follicle is characterized by its size (-0.35-0.1 mm in diameter) and a single layer of -600 cuboidal granulosa cells. Like the primordial follicle, this follicle consists of primary oocyte, granulosa cells, and fibroblasts.
(c) Secondary preantral follicle (second stage): The secondary preantral follicle is characterized by its size (-0.1-0.2 mm in diameter) and two or more layers of -2, 100 cuboidal granulosa cells that surround the primary oocyte. The secondary preantral follicle consists of three cell types: primary oocyte, granulosa cells, and thecal cells (i.e., thecal interna and externa).
(d) Secondary antral follicle (third stage): The secondary antral follicle is characterized by its size (-0.20-2.0 mm in diameter), multiple layers of -7,600-176,000 cuboidal granulosa cells, and a crescent-shaped, fluid-filled cavity called the antrum. Like the secondary preantral follicle, this follicle consists of primary oocyte, granulosa cells, and thecal cells.
(e) Selectable follicle (fourth stage): The selectable follicle is characterized by its size (-2-5 mm in diameter), multiple layers of -933,000 cuboidal granulosa cells, the presence of an antrum, and the development of folliclestimulating hormone (FSH) sensitivity. At the beginning of each menstrual cycle, a cohort of 3-11 selectable follicles is present in the ovary, from which normally only one follicle will be selected to continue ovarian growth toward the Graafian follicle and ovulation. The selectable follicle consists of the same three cell types as the secondary preantral and antral follicles.
(f) Selected preovulatory follicle (fifth stage): The selected preovulatory follicle is characterized by its size (-5-18 mm in diameter), multiple layers of -340,000 cuboidal granulosa cells, the presence of an antrum, and the development of FSH and luteinizing hormone {LH) sensitivity. The selected preovulatory follicle is the largest healthy follicle out of the cohort of 3-11 selectable follicles: it grows at a faster rate, accumulates large amounts of fluid within the antrum, contains detectable levels of FSH, and has a higher estradiol concentration. It also consists of a primary oocyte, granulosa cells, and thecal cells. The granulosa cells express FSH and LH receptors, and theca interna cells express LH receptors. The LH causes the theca interna cells to produce androstenedione, which is then transported to the granulosa cells, where it is converted to estradiol. The theca externa layer remains a highly cellular connective tissue stroma.
(g) Graafian follicle (sixth stage): The Graafian follicle is characterized by its size (-25 mm in diameter), multiple layers of -59,000,000 cuboidal granulosa cells, the presence of an antrum, FSH and LH sensitivity, and the presence of a secondary oocyte arrested in metaphase of meiosis II. The Graafian follicle consists of three cell types: secondary oocyte, granulosa cells, and thecal cells. Androstenedione is converted to estradiol as in the fifth stage of ovarian follicle growth, and the theca externa layer remains a highly cellular connective tissue stroma.
(h) Corpus luteum: The corpus luteum is a temporary endocrine gland whose formation is LH dependent. If fertilization occurs, the corpus luteum enlarges and becomes the predominant source of steroids needed to sustain pregnancy for -8 weeks. Thereafter, the placenta becomes the major source of the steroids required. If fertilization does not occur, the corpus luteum regresses and forms a corpus albicans. The corpus luteum consists of granulosa lutein and theca lutein cells.
(i) Granulosa lutein cell: This is a large (30 μm in diameter) cell with a round nucleus located toward the center of the corpus luteum. It contains predominately SER, mitochondria with tubular crista, lipid droplets, and lipochrome pigment and secretes progesterone and estradiol.
(ii) Theca lutein cell: This is a small (15 μm in diameter) cell with a round nucleus located toward the periphery of the corpus luteum. It also contains predominately SER, mitochondria with tubular crista, lipid droplets, and lipochrome pigment but secretes progesterone and androstenedione.
b. Uterine tube: The uterine tube is organized into a mucosa, muscularis layer, and a serosa.
[1] Mucosa: The mucosa of the uterine tube consists of a simple columnar epithelium and a lamina propria. The simple columnar epithelium consists of ciliated cells and secretory (peg) cells. The ciliated cells have cilia that beat toward the uterus. The secretory (Peg) cells secrete a nutrient-rich medium for the nourishment of the sperm and preimplantation embryo. The lamina propria consists of loose connective tissue.
[2] Muscularis: The muscularis layer consists of smooth muscle oriented in an inner circular layer and an outer longitudinal layer.
[3] Serosa: The serosa consists of a simple squamous epithelium (mesothelium) and a thin layer of loose connective tissue.
c. Uterus: As shown in Figure 26, the uterus is organized into in an endometrium (mucosa), myometrium (muscularis layer), and perimetrium (serosa).
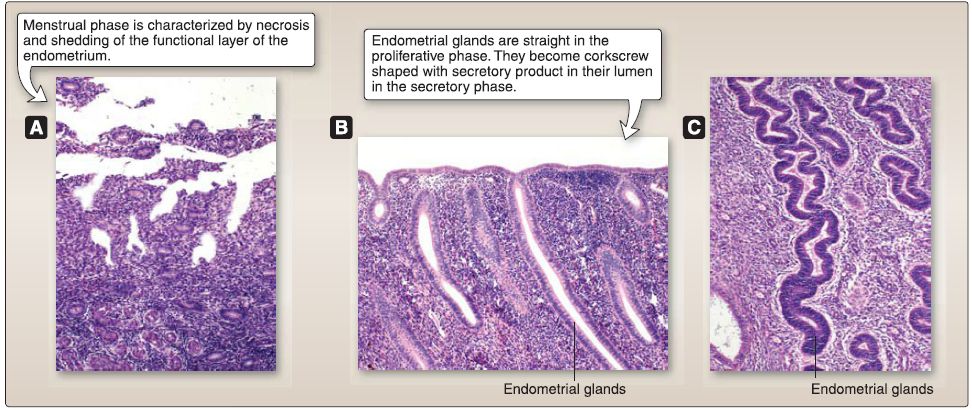 Figure 26: Uterine histology. A, Menstrual phase. B, Proliferative phase. C, Secretory phase.
Figure 26: Uterine histology. A, Menstrual phase. B, Proliferative phase. C, Secretory phase.
[1] Endometrium: The endometrium of the uterus consists of a simple columnar epithelium and an endometrial stroma {lamina propria). The simple columnar epithelium invaginates into the underlying endometrial stroma (lamina propria) to form endometrial glands, which consist of mainly secretory cells. The endometrium can be divided into a functional layer and a basal layer. The functional layer undergoes
cyclic changes each month as part of the menstrual cycle and is sloughed off each month during menses. The basal layer is responsible for the regeneration of the functional. The basal layer is never sloughed off.
[2] Myometrium: The myometrium consists of smooth muscle oriented in inner, middle, and outer layers. The inner and outer layers of smooth muscle are arranged along the long axis of the uterus proper. The middle layer of smooth muscle is arranged in a spiral manner. The middle layer of smooth muscle contains the stratum vasculare, which is highly vascular and is the source of the endometrial blood supply.
[3] Perimetrium: The perimetrium consists of mesothelium and a layer of connective tissue.
d. Menstrual cycle: The menstrual cycle is a series of five phases that repeats ideally every 28 days: menstrual, proliferative (follicular), ovulatory, secretory (luteal), and premenstrual phases.
[1] Menstrual (days 1-4): This phase is characterized by the necrosis and shedding of the functional layer of the endometrium. Spiral arterioles constrict episodically for a few days and finally constrict permanently, resulting in ischemia that leads to necrosis of endometrial glands and stroma. The spiral arterioles subsequently dilate and rupture, resulting in hemorrhage that sheds the necrotic endometrial glands and stroma.
[2] Proliferative/follicular (days 4-15): This phase is characterized by the regeneration of the functional layer of the endometrium from the devastating effects of the menstrual phase. This phase is controlled by estrogen secreted by the granulosa cells of the selected preovulatory and Graafian follicles. The epithelial cells in the basal layer of the endometrium regenerate to form straight endometrial glands with a narrow lumen.
[3] Ovulatory (days 14-16): This phase is characterized by ovulation of the secondary oocyte arrested in metaphase of meiosis II that coincides with peak levels of LH (i.e., the LH surge).
[4] Secretory/luteal (days 15-25): This phase is characterized by the secretory activity of the endometrial glands. It is controlled by progesterone secreted by the granulosa lutein cells of the corpus luteum. The endometrial glands become modified to corkscrew-shaped endometrial glands with glycogen-rich secretion product within their lumen.
[5] Premenstrual (days 25-28): This phase is characterized by ischemia due to reduced blood flow to the endometrium. It is controlled by the reduction in estrogen and progesterone as the corpus luteum involutes. As the endometrial glands begin to shrink, the spiral arterioles are compressed, thereby reducing blood flow and causing ischemic damage.
e. Cervix: The cervix is organized into a mucosa and a stromal wall (Fig. 27).
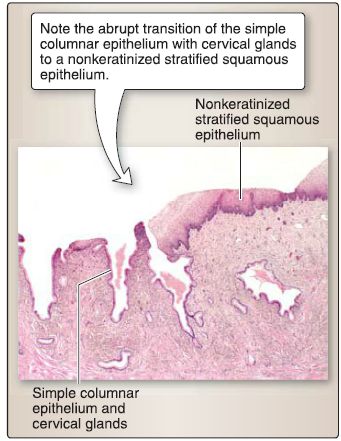
Figure 27 : Cervical histology.
[1] Mucosa: The mucosa of the cervix consists of a simple columnar epithelium or a nonkeratinized stratified squamous epithelium and a lamina propria.
(a) Simple columnar epithelium: This lines the supravaginal portion of the cervix and invaginates into the lamina propria to form cervical glands, which secrete mucus. The simple columnar epithelium and glands do not slough off during the menstrual cycle and are relatively unaffected by it. The nonkeratinized stratified squamous epithelium covers the vaginal portion of the cervix and is continuous with the vaginal epithelium.
(b) Lamina propria: This consists of a relatively thick layer of loose connective tissue.
[2] Stromal wall: The stromal wall of the cervix is predominately dense, irregular connective tissue with very little smooth muscle. This contrasts with the uterine wall, which is predominately smooth muscle. During pregnancy, the cervix becomes relatively rigid and undergoes little to no expansion. Before childbirth, the dense, irregular connective tissue undergoes extensive remodeling along with the removal of collagen fibers by macrophages. As a result, the cervix becomes pliable (cervical effacement).
f. Vagina: The vagina is organized into a mucosa, submucosa, muscularis layer, and adventitia (Fig. 28).

Figure 28: Vaginal histology
[1] Mucosa: This consists of a nonkeratinized stratified squamous epithelium that lines the lumen of the vagina and a lamina propria. The most superficial layer of the epithelium is continuously exfoliated during the menstrual cycle, but exfoliation increases during the late secretory phase and menstrual phase. The lamina propria consists of loose connective tissue with numerous elastic fibers, a rich network of blood vessels, and numerous lymphocytes and neutrophils that migrate into the epithelium.
[2] Submucosa: This consists of dense, irregular connective tissue, blood vessels, lymphatics, and some lymphatic follicles. The submucosa is not very prominent.
[3] Muscularis: This consists of an inner circular layer and an outer longitudinal layer of smooth muscle. These layers are often ill-defined bundles. The muscularis layer also contains numerous elastic fibers that contribute to the distensibility of the vagina during childbirth.
[4] Adventitia: The adventitia of the vagina consists of dense, irregular connective tissue with a rich network of elastic fibers that contribute to the distensibility of the vagina during childbirth.
5. Male reproductive system development: The components of the indifferent embryo that are remodeled to form the adult male reproductive system include the gonads, genital ducts, and primordia of external genitalia (Fig. 29). The sequence of remodeling begins with the gonads, then the genital ducts, and finally the primordia of the external genitalia .
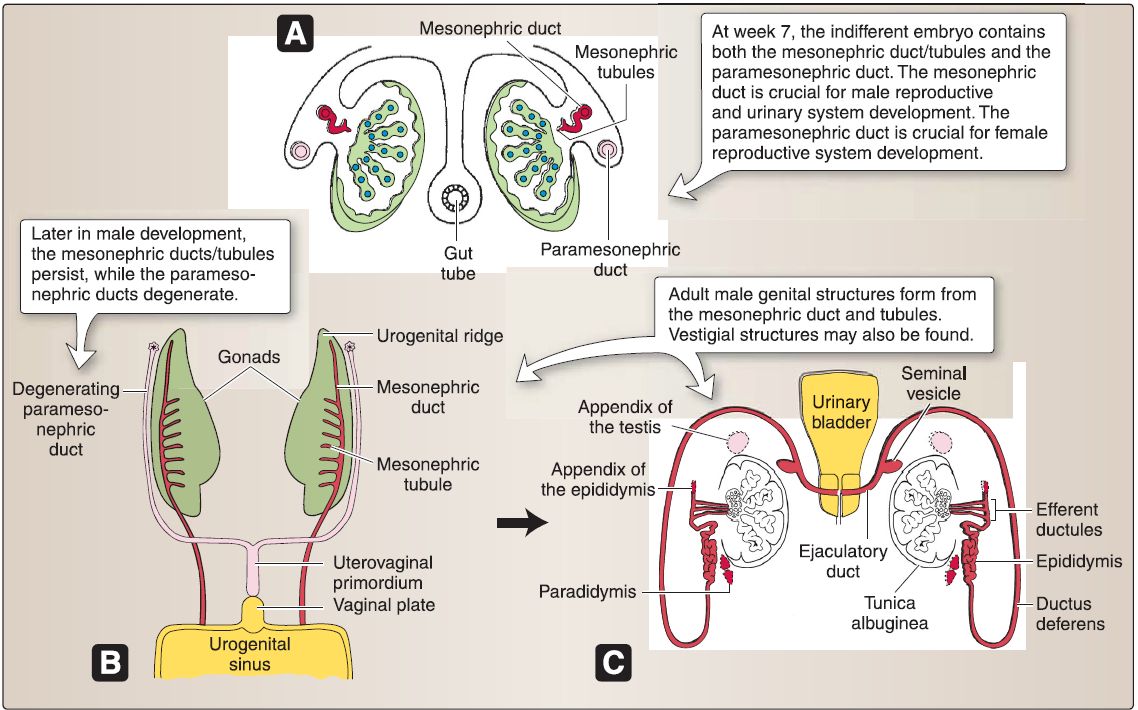 Figure 29 : Male reproductive system embryology. A, Week 7 XY gonad. B, XY gonad and genital ducts.
Figure 29 : Male reproductive system embryology. A, Week 7 XY gonad. B, XY gonad and genital ducts.
a. XY gonad: The coelomic epithelium associated with the gonadal ridge forms somatic support cells within the XY gonad. The somatic support cells express the SRY gene located on chromosome Yp11.3, which encodes for the Sry protein, a 220-amino acid transcription factor that contains a highly conserved DNAbinding region called a high-mobility group box. In response to the Sry protein, somatic support cells within the XY gonad differentiate into Sertoli cells that secrete MIF, which induces the degeneration of the paramesonephric (Mullerian) ducts. In response to signals from the Sertoli cells, mesenchymal cells within the XY gonad differentiate into Leydig cells that secrete testosterone. In the presence of Sry protein, MIF, and testosterone, the XY gonad forms the testes, and the indifferent embryo will be directed toward male characteristics. During week 7, primary sex cords develop from the gonadal ridge and incorporate primordial germ cells (XY genotype), which migrate into the XY gonad from the wall of the yolk sac. The primary sex cords extend into the medulla of the XY gonad and lose their connection with the surface epithelium as the thick tunica albuginea develops and form the secondary sex cords. These, in turn, form the seminiferous cords, tubuli recti, and the rete testes. The seminiferous cords consist of primordial germ cells surrounded by Sertoli cells and mesenchymal cells.
b. Male genital ducts: The indifferent embryo-no matter whether it possesses an XY genotype or XX genotype-contains both mesonephric {Wolffian) ducts/tubules and paramesonephric (Mullerian) ducts.
[1] Mesonephric (Wolffian): In the male, these ducts form the epididymis, ductus deferens, seminal gland, and the ejaculatory duct. The mesonephric tubules form the efferent ductules of the testes.
[2] Paramesonephric (Mullerian): In the male, these ducts degenerate due to MIF secreted by the Sertoli cells.
c. Testes descent: Initially, the testes develop in the abdomen and descend during fetal development to their final location in the scrotum (Fig. 30). This significant change in position creates a scenario in which testicular vasculature and lymphatics are pulled with the testes but trace back to the abdomen, despite that they are housed within a superficial perinea! structure. Understanding the pathway and associated structures that guide the descent will help in understanding the gross anatomy of the testes.
[1] Gubernaculum: In week 7, a ligamentous cord called the gubernaculum forms and attaches distally in the fascia of the labioscrotal swellings. The proximal end of the gubernaculum attaches to the testis (gonad).

Figure 30 :Testes descent.
[2] Processus vaginalis: Also during week 7, an invagination of peritoneum, the processus vaginalis, forms and extends inferiorly through the abdominal wall layers, ending in the labioscrotal folds. The processus vaginal is creates the pathway for testes descent that will become the inguinal canal. Recall that the inguinal canal is an oblique tunnel bound by layers of the lateral abdominal wall that has a deep and superficial ring.
[3] Descent: Testes typically descend to the deep inguinal ring by the seventh month of development. Between months 7 and 8, the testes continue through the inguinal canal, passing posterior to the processus vaginalis. Along this pathway, selective layers of the abdominal wall are pulled through to become layers of the spermatic cord and testis (see Fig. 4.8). The testes pull the ductus deferens and testicular vessels along with them, which assume a position superior and anterior to the ureters. During this descent, the gubernaculum functions as a guiding line, progressively shortening as the testes get closer to their final position in the scrotum. Eventually, the processus vaginalis is obliterated, leaving only a small remnant, the tunica vaginalis, in the scrotum, anterior to each testis. The gubernaculum remnant helps toanchor the testis within the scrotum.
6. Male reproductive system anatomy: The male reproductive system consists of paired testis, epididymis, ductus (vas) deferens, seminal glands (vesicles), prostate, and penis (Fig. 5.56). These structures collectively function to produce and carry cells and fluids that constitute semen. The male urethra is integrated into this system but described with other urinary system structures.
a. Testis: The testis (singular) is an ovoid structure that is surrounded by a thick connective tissue capsule called the tunica albuginea because of its whitish color (Fig. 31). Suspended in the scrotum, but anchored inferiorly by the gubernaculum remnant, the testis produces hormones and spermatozoa within a delicate, intricate system of tubules.
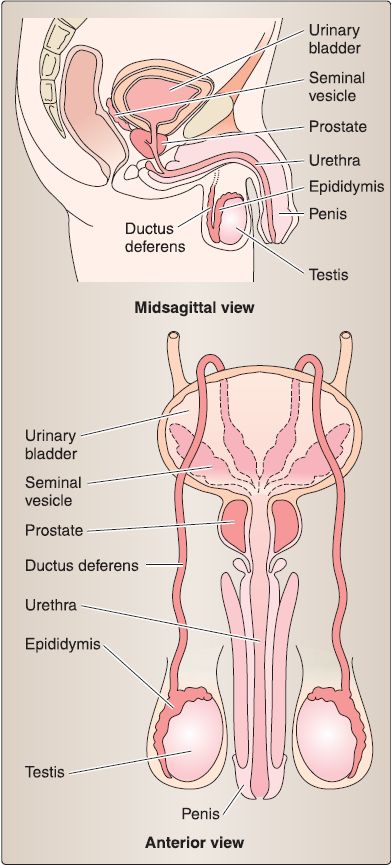
Figure 31 : Male reproductive structures.
[1] Tunica vaginalis layers: The cavity or space of the tunica vaginal is is found between visceral and parietal layers that line the testis and internal scrotum, respectively. The resulting cavity allows for movement of the testis within the scrotum.
b. Epididymis: The epididymis is a highly coiled duct -6 m in length that is divided into head, body, and tail regions ( Fig. 32). It is located posteriorly on the testis and is continuous with the ductus deferens.
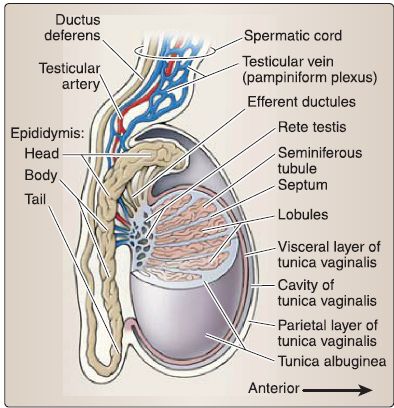
Figure 32 : Testes and epididymis.
c. Ductus (vas) deferens: The ductus deferens connects the tail of the epididymis to the ejaculatory duct (Fig. 33). It is contained within the spermatic cord and travels through the inguinal canal, anterior and superior to the ureter. At the posterior midline of the urinary bladder, it expands into the ampulla of the ductus deferens before joining the seminal gland to form the ejaculatory duct.
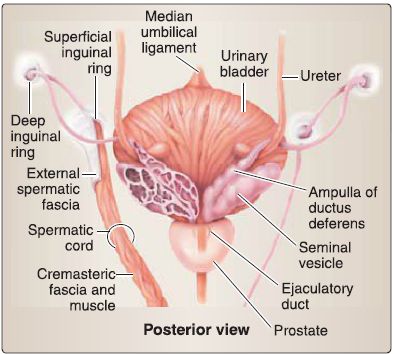
Figure 33 :Ductus deferens and seminal glands and prostate.
d. Seminal glands: Paired seminal glands sit posterior to the fund us of the urinary bladder, anterior to the rectum, and superior to the prostate (see Fig. 33). They produce an alkaline fluid that contributes to the formation of semen by way of the ejaculatory duct.
e. Prostate: Commonly referred to as the prostate gland, this walnut-sized structure is two thirds glandular and a third fibromuscular. The base of the prostate sits at the neck of the urinary bladder, while the apex is adjacent to the deep perinea! pouch fascia (fig. 33). The first main part (prostatic) of the male urethra and ejaculatory ducts travels through the substance of the prostate. It is surrounded by a fibrous capsule that holds nervous and venous plexuses of the prostate.
f. Vasculature: Blood supply to male reproductive structures can arise/drain in the abdomen or within the pelvis (Fig. 34). Veins in this region accompany arteries of the same name, unless otherwise noted.
 Figure 34: Neurovascular supply to male reproductive viscera.
Figure 34: Neurovascular supply to male reproductive viscera.
[1] Testicular arteries and veins: Testicular arteries are direct branches of the abdominal aorta that supply the testes and epididymis. The pampiniform venous plexus drains these structures and converges to form right and left testicular veins, which ultimately drain into the inferior vena cava and left renal vein, respectively.
[2] Deferential artery and vein: Typically a branch and tributary of the superior vesical artery and vein, respectively, these supply and drain the ductus deferens.
[3] Inferior vesical artery and vein: A branch of the internal iliac artery and vein, respectively, these supply and drain the ductus deferens, seminal glands, and ejaculatory ducts.
[4] Prostatic arteries and venous plexus: Prostatic arteries can arise from several internal iliac branches, including the inferior vesical, middle rectal, and internal pudendal. These arteries supply the prostate. The prostatic venous plexus, which is continuous anteriorly and superiorly with the vesical venous plexus, drains the prostate into the internal iliac veins.
[5] Dorsal artery and deep dorsal vein of penis: The dorsal artery of the penis is a branch of the internal pudendal artery that travels along the dorsum of the penis.
g. Innervation: Sympathetic and parasympathetic fibers converge to form the inferior hypogastric and pelvic plexuses (prostatic nerve plexus), which innervate male reproductive structures ( Figs. 34). Structures involved in the transport and emission of semen (ductus deferens, ejaculatory ducts, seminal glands) have extensive sympathetic innervation that controls this process.
h. Lymphatics: Lymph from male reproductive organs primarily drains into external or internal iliac nodes . However, lymph from the testis drains into lumbar lymph nodes, as it developed in the abdomen.
7. Male reproductive system histology: This includes the testis (seminiferous tubules, male duct system, and male accessory glands). Beneath the tunica albuginea, the testis is surrounded by a highly vascular layer of connective tissue called the tunica vasculosa (Fig. 35). The tunica albuginea projects connective tissue septae that divides the testis into -250 lobules, each of which contains 1-4 highly coiled seminiferous tubules. Each seminiferous tubule ends as a straight tubule, all of which empty into the rete testis, which is an anastomosing labyrinth of channels. The connective tissue septae are continuous with the loose connective tissue between the seminiferous tubules where the Leydig (interstitial) cells that secrete testosterone are located.
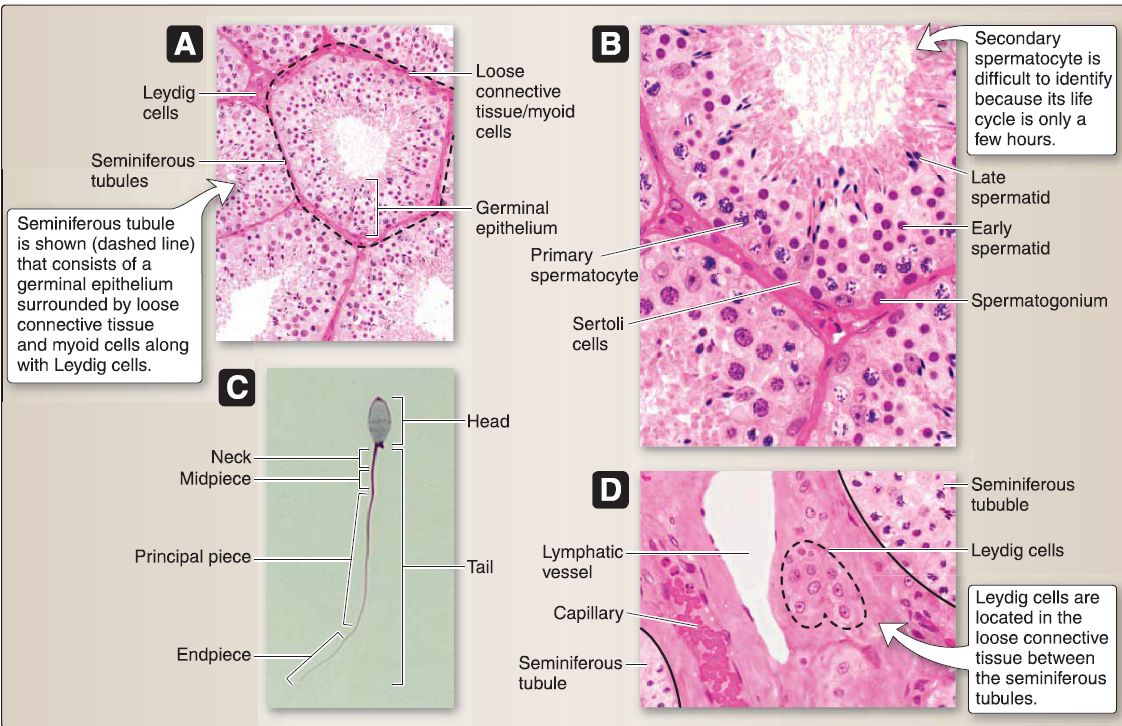 Figure 35 : Testicular histology. A, Seminiferous tubules. B, Germinal epithelium. C, Spermatozoon. D, Leydig cells.
Figure 35 : Testicular histology. A, Seminiferous tubules. B, Germinal epithelium. C, Spermatozoon. D, Leydig cells.
a. Seminiferous tubules: These consist of a germinal epithelium surrounded by loose connective tissue and myoid cells. The germinal epithelium (a complex stratified epithelium) comprises two basic cell types: Sertoli and spermatogenic cells.
[1] Sertoli cell: This tall, columnar cell has an oval or triangular, basally or centrally located nucleus with one or more deep infoldings. It has unusually ruffled apical and lateral surfaces,
as these surround the developing spermatogenic cells. The Sertoli cell extends the full thickness of the germinal epithelium and contains RER, polyribosomes, a Golgi complex, SER, mitochondria, annulate lamellae, lysosomes, vesicles, lipid droplets, glycogen, microtubules, a 7-9-nm filament sheath that surrounds the nucleus, and crystalloid inclusion bodies (of Charcot-Bottcher) that consist of 15-nm filament bundles.
(a) Structure: Sertoli cells are joined to each other by a unique zonula occludens (tight junction) associated with SER and actin filaments. This zonula occludens establishes two compartments within the germinal epithelium: basal and luminal. The basal compartment contains spermatogonia and early primary spermatocytes, and the luminal compartment contains more mature spermatocytes and spermatids. The zonula occludens also establishes the blood-testis barrier, which creates a pristine fluid environment for the more mature spermatocytes and spermatids and protects them (as well as genetically dissimilar cells) from the immune system. (b} Functions: The Sertoli cell has these important functions: it provides mechanical and nutritional support for developing spermatogenic cells, phagocytizes excess cytoplasm discarded by spermatids, forms the blood-testes barrier, secretes inhibin that inhibits release of FSH from the adenohypophysis, secretes MIF during fetal development that inhibits development of the paramesonephric duct in a genotypic XY fetus, synthesizes androgen-binding protein (ABP) that binds testosterone so that the high levels necessary for spermatogenesis to occur are present in the seminiferous tubules, and it possesses FSH receptors (G-protein-linked receptors) so that FSH from the adenohypophysis stimulates spermatogenesis and synthesis of ABP.
[2] Spermatogenic cells: These "male germ cells" are transforming from type A spermatogonia ➔ spermatozoa, a process called spermatogenesis. Spermatogenic cells migrate from the basal to the luminal compartment of the germinal epithelium as they undergo spermatogenesis. They consist of a number of cell types: dark and pale type A spermatogonia, type B spermatogonium, primary and secondary spermatocytes, spermatid (early and late stages), and spermatozoon.
(a) Spermatozoon: The spermatozoon undergoes maturation in the epididymis and capacitation in the female reproductive tract so that it is capable of delivering its (23, 1 N) complement of chromosomal material to the secondary oocyte. The spermatozoon consists of head and tail regions. The tail is further divided into a neck, midpiece, principal piece, and end piece.
b. Leydig cell: This large, polygon-shaped cell has a round, centrally located nucleus and contains RER, polyribosomes, a Golgi complex, prominent SER, mitochondria with tubular cristae, lipid droplets, lysosomes, glycogen, lipofuscin pigment, and crystals of Reinke. The Leydig cell is located in the loose connective tissue between the seminiferous tubules. It possesses LH receptors so that LH from the adenohypophysis stimulates testosterone secretion.
[1] Testosterone: This steroid hormone secreted by Leydig cells plays a role in fetal development of the epididymis, ductus deferens, seminal gland, and ejaculatory duct (dihydrotestosterone is essential in the fetal development of the penis and scrotum [external genitalia] and prostate); spermatogenesis; function of the prostate, seminal gland, and bulbourethral glands; appearance of secondary sex characteristics; closure of the epiphyseal growth plate; increase in muscle mass; lipid metabolism (testosterone supplementation increases highdensity lipoprotein and decreases low-density lipoprotein); and stimulation of cartilage growth.
c. Male duct system: The spermatozoa travel from the seminiferous tubules ➔ straight tubules ➔ rete testis all of which are located inside the testis. The spermatozoa then travel from the efferent ductules ➔ epididymis ➔ ductus deferens ➔ ejaculatory duct
➔ urethra, all of which are located outside the testis.
[1] Efferent ductules: The -20 efferent ductules consist of an epithelium, loose connective tissue, and a smooth muscle layer. The epithelium consists of alternating patches of ciliated simple columnar epithelium and unciliated simple cuboidal epithelium, giving the luminal surface an irregular or saw-toothed appearance.
[2] Epididymis: This is the site where spermatozoa maturation (i.e., develop motility) and short-term storage occurs (Fig. 36). It consists of an epithelium, loose connective
tissue, and a smooth muscle layer. The epithelium consists of a pseudostratified columnar epithelium with tall, columnar principal and basal cells (stem cells). The pseudostratified columnar epithelium gives the luminal surface a smooth appearance in contrast to the irregular appearance of the efferent ductules.
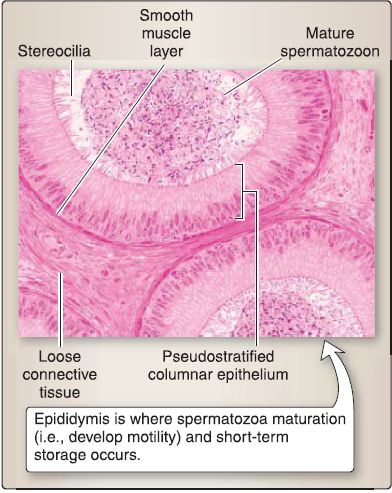
Figure 36 : Epididymis histology.
(a) Principal cell functions: This cell reabsorbs -20% of the testicular fluid (efferent ductules reabsorb -80%); phagocytizes degenerating spermatozoa or spermatid residual bodies not phagocytized by Sertoli cells; and secretes glycoproteins, sialic acid, and glycerophosphocholine, which aid in spermatozoa maturation.
[3] Ductus (vas) deferens: This consists of an epithelium,loose connective tissue, and a muscle layer. The epithelium consists of a pseudostratified columnar epithelium with tall, columnar principal and basal cells (stem cells).
[4] Ejaculatory duct: This consists of an epithelium and the fibromuscular stroma of the prostate because the ejaculatory duct passes through the prostate. The epithelium consists of a pseudostratified columnar epithelium.
d. Male accessory glands: These include the seminal vesicle, bulbourethral, and prostate glands. Collectively, they produce secretions that contribute to the formation of semen or function to lubricate the male urethra.
[1] Seminal vesicle: This gland is organized into a pseudostratified columnar epithelium, loose connective tissue, a muscle layer, and a capsule. The epithelium consists of secretory and basal (stem) cells. The secretory cell secretes a pale yellow, viscous material called seminal fluid that contains fructose (the principal metabolic substrate for sperm), other sugars, choline, proteins, amino acids, ascorbic acid, citric acid, and prostaglandins. The seminal fluid accounts for 70% of the volume of the ejaculated semen.
[2] Bulbourethral: This gland is organized into a simple cuboidal epithelium, loose connective tissue, and a capsule. The epithelium produces a clear, mucus-like, slippery fluid, which contains galactose, galactosamine, galacturonic acid, sialic acid, and methylpentose. This fluid makes up a major portion of the preseminal fluid (pre-ejaculate) and probably serves to lubricate the spongy urethra.
[3] Prostate: This gland is organized into a pseudostratified columnar epithelium, fibromuscular stroma, and a capsule (Fig. 37). The epithelium consists of secretory, neuroendocrine, and basal (stem) cells. The secretory cell secretes a clear, slightly alkaline (pH 7.29) prostatic fluid that contains citric acid, prostatic acid phosphatase, prostaglandins, fibrinolysin, and prostate-specific antigen (PSA). This fluid may precipitate or calcify within the lumen of the tubuloalveolar glands and form prostatic concretions, which increase with age.
(a) Neuroendocrine cell: This cell secretes serotonin, somatostatin, calcitonin, and bombesin. It regulates the growth, differentiation, and secretory function of the prostate.
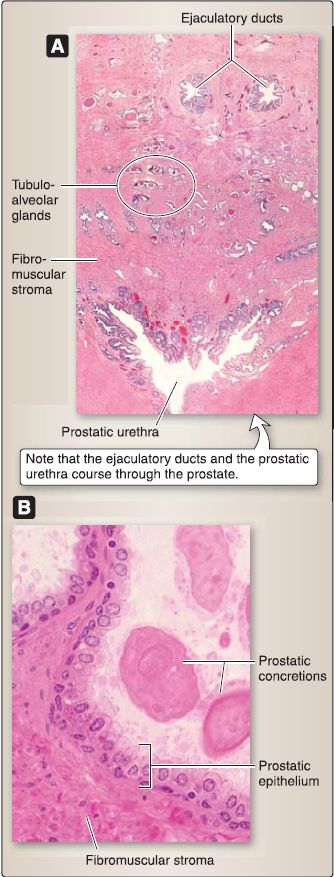
Figure 37: Prostate histology. A, Low magnification showing ducts and urethra. B, High magnification.
 الاكثر قراءة في علم التشريح
الاكثر قراءة في علم التشريح
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


