


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-12-2015
Date: 31-3-2021
Date: 28-3-2021
|
Ribozymes Have Various Catalytic Activities
KEY CONCEPTS
- By changing the substrate binding site of a group I intron,it is possible to introduce alternative sequences that interact with the reactive G.
- The reactions follow classical enzyme kinetics with a low catalytic rate.
- Reactions using 2′–OH bonds could have been the basis for evolving the original catalytic activities in RNA.
- Synthetic RNA constructs that have RNA polymerase activity have been constructed.
The catalytic activity of group I introns was discovered by virtue of their ability to autosplice, but they are able to undertake other catalytic reactions in vitro. All of these reactions are based on transesterifications. These reactions will now be analyzed in terms of their relationship to the splicing reaction itself.
The catalytic activity of a group I intron is conferred by its ability to generate particular secondary and tertiary structures that create active sites that are equivalent to the active sites of conventional (proteinaceous) enzymes. FIGURE 1 illustrates the splicing reaction in terms of these sites .
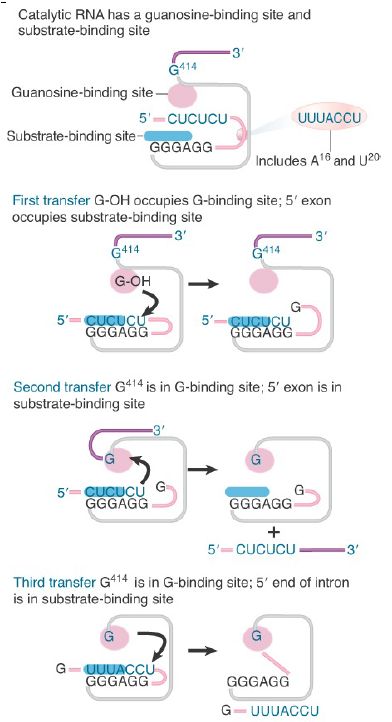
FIGURE 1. Excision of the group I intron in Tetrahymena rRNA occurs by successive reactions between the occupants of the guanosine-binding site and the substrate-binding site. The left exon is pink, and the right exon is purple.
The substrate-binding site is formed from the P1 helix, in which the 3′ end of the first intron base pairs with the IGS. A guanosinebinding site is formed by sequences in P7. This site may be occupied either by a free exogenous guanosine nucleotide (exoG) or by the ΩG residue (position 414 in Tetrahymena). In the first transfer reaction, the guanosine-binding site is occupied by free guanosine nucleotide. Following release of the intron, it is occupied by ΩG. The second transfer releases the joined exons. The third transfer creates the circular intron.
Binding to the substrate involves a change of conformation. Before substrate binding, the 5′ end of the IGS is close to P2 and P8; after binding, when it forms the P1 helix, it is close to conserved bases that lie between P4 and P5. The reaction is visualized by contacts that are detected in the secondary structure in FIGURE 2. In the tertiary structure, the two sites alternatively contacted by P1 are 37 Å apart, which implies a substantial movement in the position of P1.
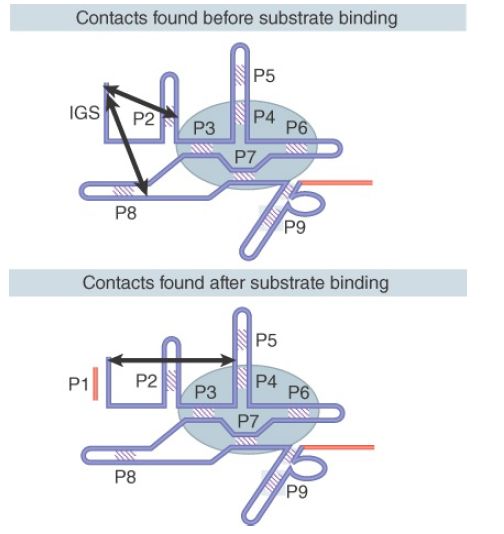
FIGURE 2. The position of the IGS in the tertiary structure changes when P1 is formed by substrate binding.
Additional enzymatic reactions that can be performed by Tetrahymena group I introns are characterized in FIGURE 3. The ribozyme can function as a sequence-specific endoribonuclease by utilizing the ability of the IGS to bind complementary sequences. In this example, it binds an external substrate containing the sequence CUCU, instead of binding the analogous sequence that is usually contained at the end of the 5′ exon. A guanosine-containing nucleotide is present in the G-binding site and attacks the CUCU sequence in precisely the same way that the exon is usually attacked in the first transfer reaction. This cleaves the target sequence into a 5′ molecule that resembles the 5′ exon and a 3′ molecule that bears a terminal G residue.
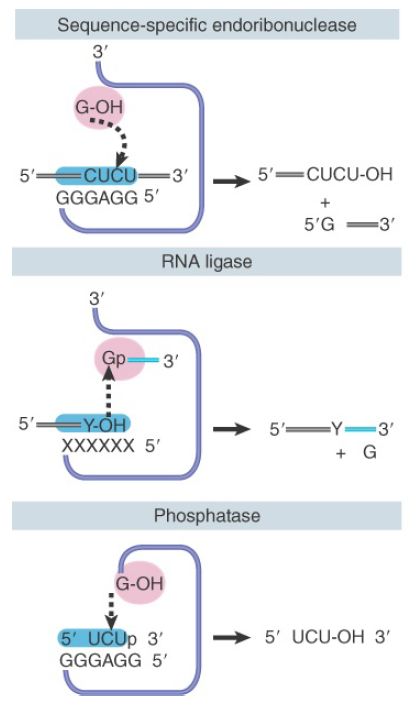
FIGURE 3.Catalytic reactions of the ribozyme involve transesterifications between a group in the substrate-binding site and a group in the G-binding site.
By mutating the IGS element, it is possible to change the specificity of the ribozyme so that it recognizes sequences complementary to the new sequence at the IGS region. This alteration of the IGS to change the specificity of the substrate-binding site enables other RNA targets to be processed by the ribozyme, which can also be used to perform RNA ligase reactions. An RNA terminating in a 3′–OH is bound in the substrate site, and an RNA terminating in a 5′–G residue is bound in the G-binding site. An attack by the hydroxyl on the phosphate bond connects the two RNA molecules, with the loss of the G residue.
The phosphatase reaction is not directly related to the splicing transfer reactions. An oligonucleotide sequence that is complementary to the IGS and terminates in a 3′–phosphate can be attacked by the ΩG. The phosphate is transferred to the ΩG, and an oligonucleotide with a free 3′–OH end is then released. The phosphate can then be transferred either to an oligonucleotide terminating in 3′–OH (effectively reversing the reaction) or even to water, releasing inorganic phosphate and completing an authentic phosphatase reaction.
The reactions catalyzed by RNA can be characterized in the same way as classical enzymatic reactions in terms of Michaelis–Menten kinetics.
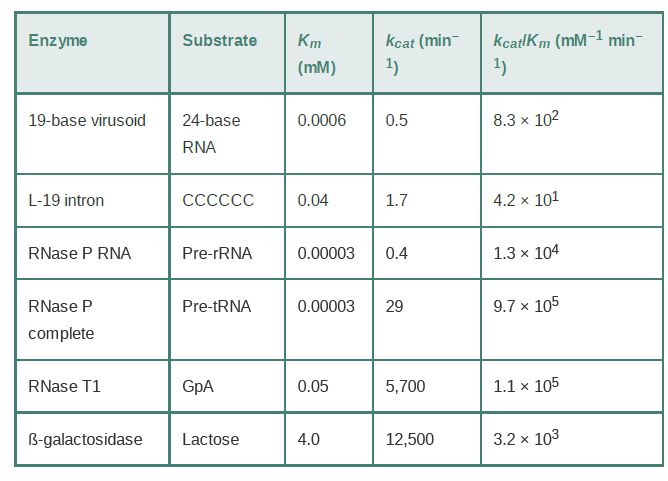
TABLE .1 analyzes the reactions catalyzed by RNA. The Km values for RNA-catalyzed reactions are low and therefore imply that the RNA can bind its substrate with high specificity. However, the turnover numbers (kcat ) for RNA-catalyzed reactions are low, which reflects a low catalytic rate. Comparing the specificity constants (kcat /Km ) of ribozymes with enzymes in TABLE 21.9 reveals that enzymes and ribozymes are comparable in terms of catalytic efficiency.
TABLE 21.1 Reactions catalyzed by RNA have the same features as those catalyzed by proteins, although the rate is slower. The Km gives the concentration of substrate required for half-maximum velocity; this is an inverse measure of the affinity of the enzyme for substrate. The kcat gives the turnover number, and the specificity constant is represented by (kcat /Km ).
A powerful extension of the activities of ribozymes has been made with the discovery that they can be regulated by ligands . These cis-acting regulatory RNA regions are called riboswitches. In almost all riboswitches, a conformational change determines the on or off state of the switch. This conformational change then alters either transcriptional attenuation or translational initiation. One notable exception is the riboswitch regulating the glmS gene, which encodes glucosamine- 6-phosphate (GlcN6P) synthase in Gram-positive bacteria. This is a negative feedback mechanism that forms a self-cleavingribozyme in the presence of GlcN6P, the product of GlcN6P synthase.
If an active center is a surface that exposes a series of active groups in a fixed relationship, it is possible to understand how RNA is capable of providing a catalytic center. In a protein, the active groups are provided by the side chains of the amino acids. The amino acid side chains have appreciable variety, including positive and negative ionic groups and hydrophobic groups. In RNA, the available moieties are more restricted, consisting primarily of the exposed groups of bases. Short regions of RNA are held in a particular secondary/tertiary conformation, providing an active surface and maintaining an environment in which bonds can be broken and formed. It seems inevitable that the interaction between the RNA catalyst and the RNA substrate will rely on base pairing to
create the active environment. Divalent cations (usually Mg+2 ) play an important role in structure, typically being present at the active site where they coordinate the positions of the various groups. Divalent metal cations also play a direct role in the endonucleolytic activity of virusoid ribozymes .
The evolutionary implications of these discoveries are intriguing. The “split personality” of the genetic apparatus—in which RNA is present in all components but proteins undertake catalytic reactions —has always been puzzling. It seems unlikely that the very first replicating systems could have contained both nucleic acid and protein. However, suppose that the first systems contained only a self-replicating nucleic acid with primitive catalytic activities—just those needed to make and break phosphodiester bonds. If it is also assumed that the involvement of 2′–OH bonds in current splicing reactions is derived from these primitive catalytic activities,
this can be taken as support of the suggestion that the original nucleic acid was RNA, because DNA lacks the 2′–OH group, and therefore could not undertake such reactions. Several experiments utilizing synthetic RNA support the possibility RNA can indeed direct its own synthesis. In early experiments, RNA ligase activity was isolated from a large pool of random RNA sequences. Further engineering of these RNA ligase ribozymes led to development of ribozymes capable of performing template-based synthesis of RNA polynucleotides over 200 nucleotides in length. If ribozymes were the first RNA polymerase molecules in the natural world, proteins could have been added for their ability to stabilize the RNA structure. The greater versatility of proteins then could have allowed them to take over catalytic reactions, leading eventually to the complex and sophisticated apparatus of modern gene expression.



|
|
|
|
كيف تعزز نمو الشعر الصحي؟
|
|
|
|
|
|
|
10 فحوصات مهمة يجب القيام بها لسيارتك قبل الصيف
|
|
|
|
|
|
قسم التربية والتعليم ينظّم جلسةً حوارية لملاكه حول تأهيل المعلّمِين الجدد
|
|
|
|
جامعة العميد تحدّد أهداف إقامة حفل التخرّج لطلبتها
|
|
|
|
جامعة العميد تحتفي بتخرّج الدفعة الثانية من طلبة كلّية الطبّ
|
|
|
|
قسم الشؤون الفكريّة يشارك في المؤتمر العلمي الدولي الخامس في النجف الأشرف
|