


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-4-2019
Date: 5-5-2019
Date: 1-1-2017
|
The electron was discovered by J.J. Thomson in 1897. The existence of protons was also known, as was the fact that atoms were neutral in charge. Since the intact atom had no net charge and the electron and proton had opposite charges, the next step after the discovery of subatomic particles was to figure out how these particles were arranged in the atom. This is a difficult task because of the incredibly small size of the atom. Therefore, scientists set out to design a model of what they believed the atom could look like. The goal of each atomic model was to accurately represent all of the experimental evidence about atoms in the simplest way possible.
Following the discovery of the electron, J.J. Thomson developed what became known as the "plum pudding" model in 1904. Plum pudding is an English dessert similar to a blueberry muffin. In Thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge like blueberries stuck into a muffin. The positive matter was thought to be jelly-like or a thick soup. The electrons were somewhat mobile. As they got closer to the outer portion of the atom, the positive charge in the region was greater than the neighboring negative charges and the electron would be pulled back more toward the center region of the atom.
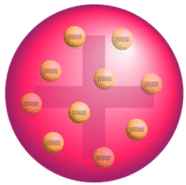
Figure 1 : The "plum pudding" model.
However, this model of the atom soon gave way to a new model developed by New Zealander Ernest Rutherford (1871 - 1937) about five years later. Thomson did still receive many honors during his lifetime, including being awarded the Nobel Prize in Physics in 1906 and a knighthood in 1908.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|