


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-12-2019
Date: 20-7-2018
Date: 5-12-2019
|
Maltose occurs to a limited extent in sprouting grain. It is formed most often by the partial hydrolysis of starch and glycogen. In the manufacture of beer, maltose is liberated by the action of malt (germinating barley) on starch; for this reason, it is often referred to as malt sugar. Maltose is about 30% as sweet as sucrose. The human body is unable to metabolize maltose or any other disaccharide directly from the diet because the molecules are too large to pass through the cell membranes of the intestinal wall. Therefore, an ingested disaccharide must first be broken down by hydrolysis into its two constituent monosaccharide units. In the body, such hydrolysis reactions are catalyzed by enzymes such as maltase. The same reactions can be carried out in the laboratory with dilute acid as a catalyst, although in that case the rate is much slower, and high temperatures are required. Whether it occurs in the body or a glass beaker, the hydrolysis of maltose produces two molecules of D-glucose.
Maltose is a reducing sugar. Thus, its two glucose molecules must be linked in such a way as to leave one anomeric carbon that can open to form an aldehyde group. The glucose units in maltose are joined in a head-to-tail fashion through an α-linkage from the first carbon atom of one glucose molecule to the fourth carbon atom of the second glucose molecule (that is, an α-1,4-glycosidic linkage; see Figure 1). The bond from the anomeric carbon of the first monosaccharide unit is directed downward, which is why this is known as an α-glycosidic linkage. The OH group on the anomeric carbon of the second glucose can be in either the α or the β position, as shown in Figure 1.
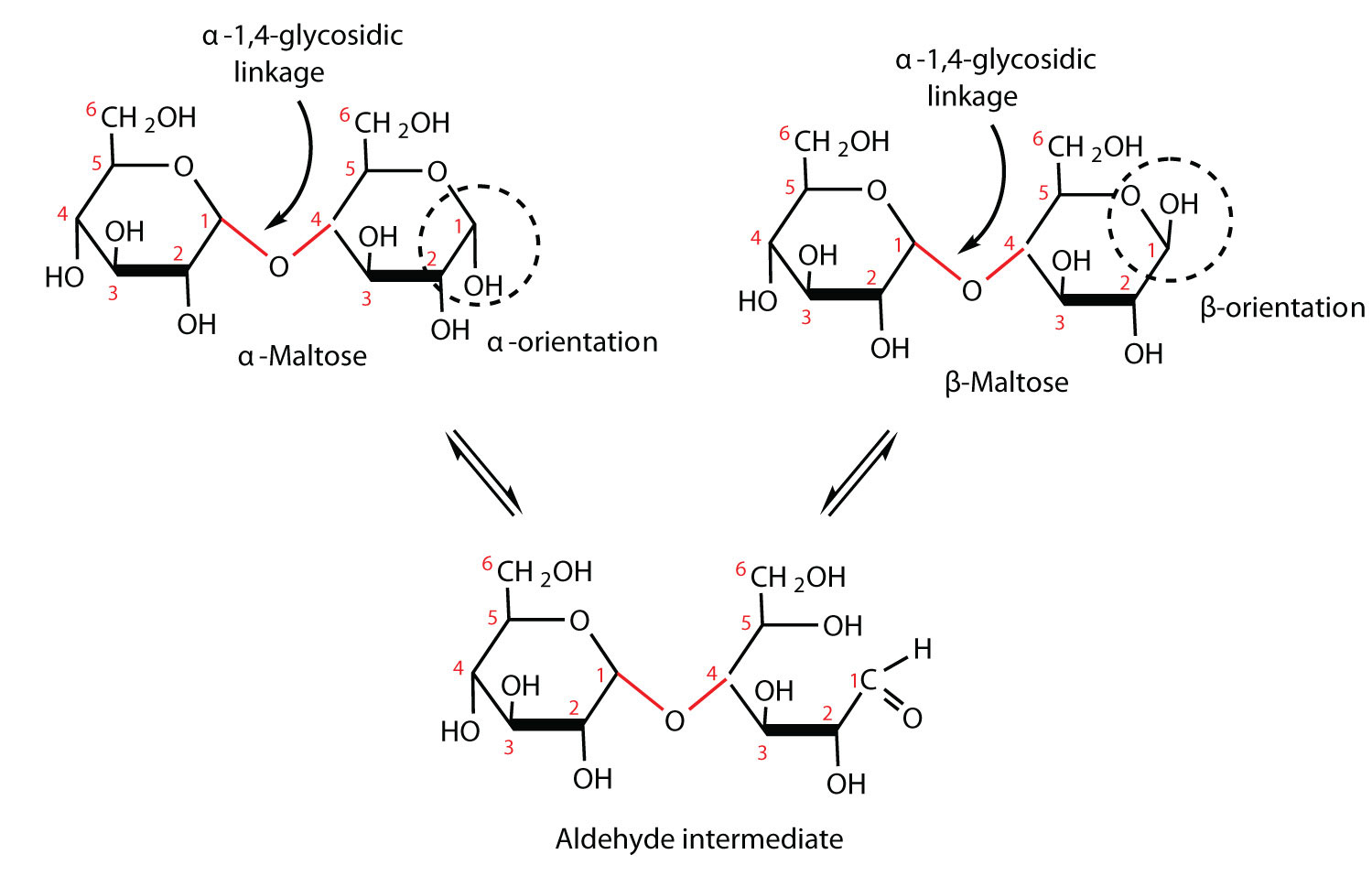
Figure 1 An Equilibrium Mixture of Maltose Isomers



|
|
|
|
للتخلص من الإمساك.. فاكهة واحدة لها مفعول سحري
|
|
|
|
|
|
|
العلماء ينجحون لأول مرة في إنشاء حبل شوكي بشري وظيفي في المختبر
|
|
|
|
|
|
|
قسم العلاقات العامّة ينظّم برنامجاً ثقافياً لوفد من أكاديمية العميد لرعاية المواهب
|
|
|