


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date:
Date: 8-10-2018
Date: 12-7-2018
|
The most common reaction of ethers is cleavage of the C–O bond by strong acids. This may occur by SN1 or E1 mechanisms for 3º-alkyl groups or by an SN2 mechanism for 1º-alkyl groups. Some examples are shown in the following diagram. The conjugate acid of the ether is an intermediate in all these reactions, just as conjugate acids were intermediates in certain alcohol reactions.
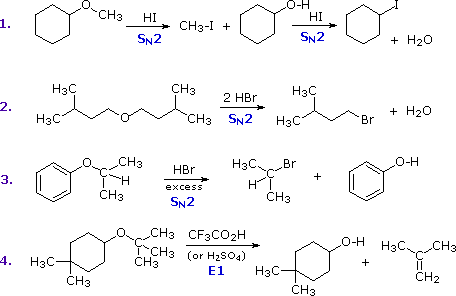
The first two reactions proceed by a sequence of SN2 steps in which the iodide or bromide anion displaces an alcohol in the first step, and then converts the conjugate acid of that alcohol to an alkyl halide in the second. Since SN2 reactions are favored at least hindered sites, the methyl group in example #1 is cleaved first. The 2º-alkyl group in example #3 is probably cleaved by an SN2 mechanism, but the SN1 alternative cannot be ruled out. The phenol formed in this reaction does not react further, since SN2, SN1 and E1 reactions do not take place on aromatic rings. The last example shows the cleavage of a 3º-alkyl group by a strong acid.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|