


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-8-2018
Date: 11-7-2018
Date: 24-10-2019
|
Some common substituents, like NO2, Br, and Cl, can be named this way when it is attached to a phenyl group. Long chain carbons attached can also be named this way. The general format for this kind of naming is:
(positions of substituents (if >1)- + # (di, tri, ...) + substituent)n + benzene.
For example, chlorine (Cl) attached to a phenyl group would be named chlorobenzene (chloro + benzene). Since there is only one substituent on the benzene ring, we do not have to indicate its position on the benzene ring (as it can freely rotate around and you would end up getting the same compound.)
.png?revision=1)
.png?revision=1)
Figure 8. Example of simple benzene naming with chlorine and NO2 as substituents.
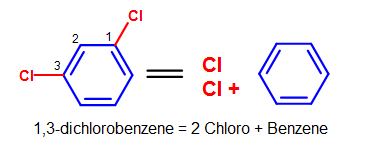
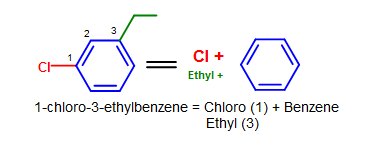
Figure 9. More complicated simple benzene naming examples - Note that standard nomenclature priority rules are applied here, causing the numbering of carbons to switch.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
مكتب السيد السيستاني يعزي أهالي الأحساء بوفاة العلامة الشيخ جواد الدندن
|
|
|