
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-7-2020
Date: 27-3-2017
Date: 23-2-2018
|
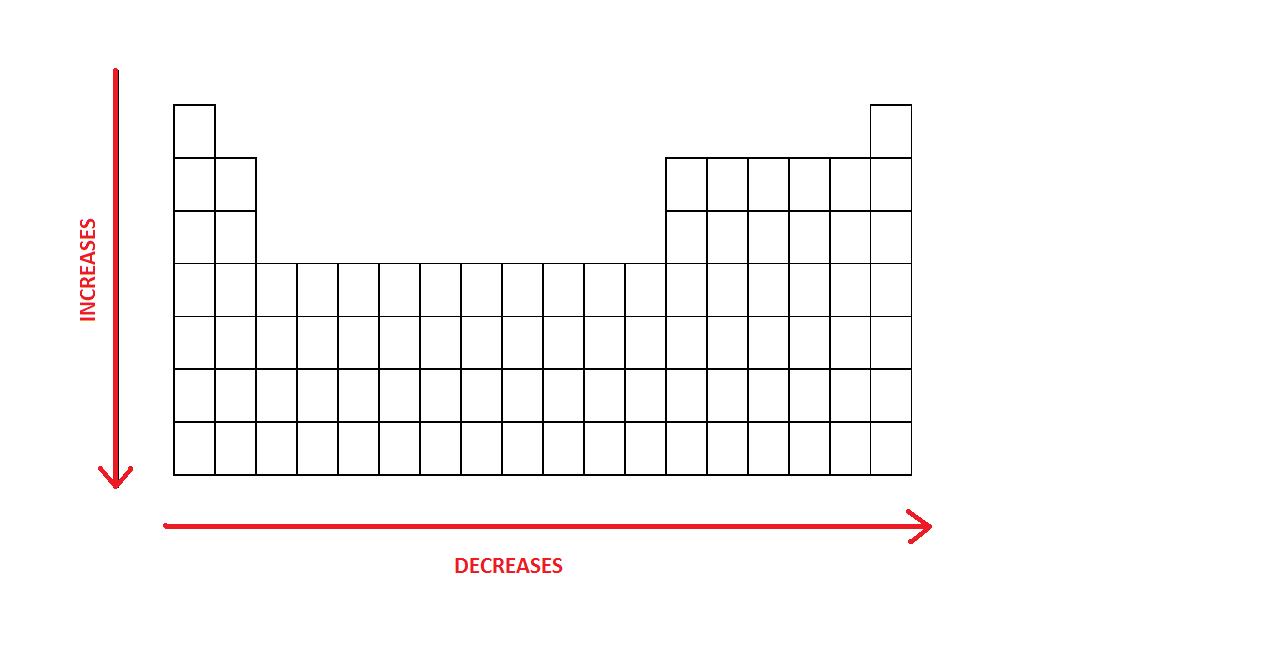
Figure 1: Periodic Trend in atomic radii
Vertical Trend
The radius of atoms increases as you go down a certain group.
Horizontal Trend
The size of an atom will decrease as you move from left to the right of a period.
EXCEPTIONS: Because the electrons added in the transition elements are added in the inner electron shell and at the same time, the outer shell remains constant, the nucleus attracts the electrons inward. The electron configuration of the transition metals explains this phenomenon. This is why Ga is the same size as its preceding atom and why Sb is slightly bigger than Sn.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|