
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Heat engines; the first law
المؤلف:
Richard Feynman, Robert Leighton and Matthew Sands
المصدر:
The Feynman Lectures on Physics
الجزء والصفحة:
Volume I, Chapter 44
2024-06-06
1758
The determination of the relationships among the various properties of materials, without knowing their internal structure, is the subject of thermodynamics. Historically, thermodynamics was developed before an understanding of the internal structure of matter was achieved.
To give an example: we know from the kinetic theory that the pressure of a gas is caused by molecular bombardment, and we know that if we heat a gas, so that the bombardment increases, the pressure must increase. Conversely, if the piston in a container of the gas is moved inward against the force of bombardment, the energy of the molecules bombarding the piston will increase, and consequently the temperature will increase. So, on the one hand, if we increase the temperature at a given volume, we increase the pressure. On the other hand, if we compress the gas, we will find that the temperature will rise. From the kinetic theory, one can derive a quantitative relationship between these two effects, but instinctively one might guess that they are related in some necessary fashion which is independent of the details of the collisions.
Let us consider another example. Many people are familiar with this interesting property of rubber: If we take a rubber band and pull it, it gets warm. If one puts it between his lips, for example, and pulls it out, he can feel a distinct warming, and this warming is reversible in the sense that if he relaxes the rubber band quickly while it is between his lips, it is distinctly cooled. That means that when we stretch a rubber band it heats, and when we release the tension of the band it cools. Now our instincts might suggest that if we heated a band, it might pull: that the fact that pulling a band heats it might imply that heating a band should cause it to contract. And, in fact, if we apply a gas flame to a rubber band holding a weight, we will see that the band contracts abruptly (Fig. 44–1). So it is true that when we heat a rubber band it pulls, and this fact is definitely related to the fact that when we release the tension of it, it cools.
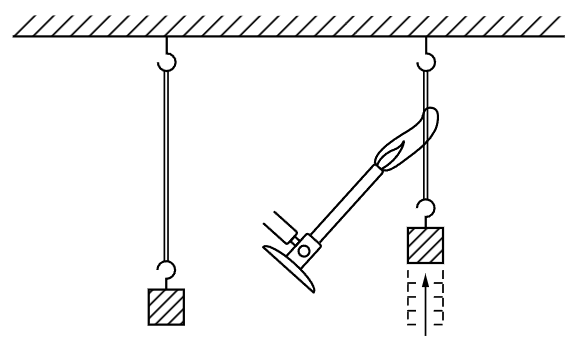
Fig. 44–1. The heated rubber band.
The internal machinery of rubber that causes these effects is quite complicated. We will describe it from a molecular point of view to some extent, although our main purpose in this chapter is to understand the relationship of these effects independently of the molecular model. Nevertheless, we can show from the molecular model that the effects are closely related. One way to understand the behavior of rubber is to recognize that this substance consists of an enormous tangle of long chains of molecules, a kind of “molecular spaghetti,” with one extra complication: between the chains there are cross-links—like spaghetti that is sometimes welded together where it crosses another piece of spaghetti—a grand tangle. When we pull out such a tangle, some of the chains tend to line up along the direction of the pull. At the same time, the chains are in thermal motion, so they hit each other continually. It follows that such a chain, if stretched, would not by itself remain stretched, because it would be hit from the sides by the other chains and other molecules, and would tend to kink up again. So the real reason why a rubber band tends to contract is this: when one pulls it out, the chains are lengthwise, and the thermal agitations of the molecules on the sides of the chains tend to kink the chains up, and make them shorten. One can then appreciate that if the chains are held stretched and the temperature is increased, so that the vigor of the bombardment on the sides of the chains is also increased, the chains tend to pull in, and they are able to pull a stronger weight when heated. If, after being stretched for a time, a rubber band is allowed to relax, each chain becomes soft, and the molecules striking it lose energy as they pound into the relaxing chain. So the temperature falls.
We have seen how these two processes, contraction when heated and cooling during relaxation, can be related by the kinetic theory, but it would be a tremendous challenge to determine from the theory the precise relationship between the two. We would have to know how many collisions there were each second and what the chains look like, and we would have to take account of all kinds of other complications. The detailed mechanism is so complex that we cannot, by kinetic theory, really determine exactly what happens; still, a definite relation between the two effects we observe can be worked out without knowing anything about the internal machinery!
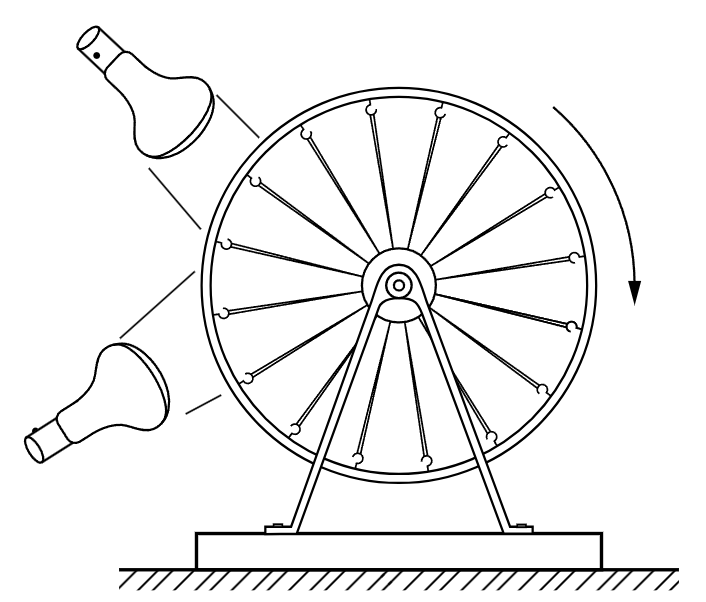
Fig. 44–2. The rubber band heat engine.
The whole subject of thermodynamics depends essentially upon the following kind of consideration: because a rubber band is “stronger” at higher temperatures than it is at lower temperatures, it ought to be possible to lift weights, and to move them around, and thus to do work with heat. In fact, we have already seen experimentally that a heated rubber band can lift a weight. The study of the way that one does work with heat is the beginning of the science of thermodynamics. Can we make an engine which uses the heating effect on a rubber band to do work? One can make a silly looking engine that does just this. It consists of a bicycle wheel in which all the spokes are rubber bands (Fig. 44–2). If one heats the rubber bands on one side of the wheel with a pair of heat lamps, they become “stronger” than the rubber bands on the other side. The center of gravity of the wheel will be pulled to one side, away from the bearing, so that the wheel turns. As it turns, cool rubber bands move toward the heat, and the heated bands move away from the heat and cool, so that the wheel turns slowly so long as the heat is applied. The efficiency of this engine is extremely low. Four hundred watts of power pour into the two lamps, but it is just possible to lift a fly with such an engine! An interesting question, however, is whether we can get heat to do the work in more efficient ways.
In fact, the science of thermodynamics began with an analysis, by the great engineer Sadi Carnot, of the problem of how to build the best and most efficient engine, and this constitutes one of the few famous cases in which engineering has contributed fundamentally to physical theory. Another example that comes to mind is the more recent analysis of information theory by Claude Shannon. These two analyses, incidentally, turn out to be closely related.
Now the way a steam engine ordinarily operates is that heat from a fire boils some water, and the steam so formed expands and pushes on a piston which makes a wheel go around. So the steam pushes the piston—what then? One has to finish the job: a stupid way to complete the cycle would be to let the steam escape into the air, for then one has to keep supplying water. It is cheaper—more efficient—to let the steam go into another box, where it is condensed by cool water, and then pump the water back into the boiler, so that it circulates continuously. Heat is thus supplied to the engine and converted into work. Now would it be better to use alcohol? What property should a substance have so that it makes the best possible engine? That was the question to which Carnot addressed himself, and one of the by-products was the discovery of the type of relationship that we have just explained above.
The results of thermodynamics are all contained implicitly in certain apparently simple statements called the laws of thermodynamics. At the time when Carnot lived, the first law of thermodynamics, the conservation of energy, was not known. Carnot’s arguments were so carefully drawn, however, that they are valid even though the first law was not known in his time! Some time afterwards, Clapeyron made a simpler derivation that could be understood more easily than Carnot’s very subtle reasoning. But it turned out that Clapeyron assumed, not the conservation of energy in general, but that heat was conserved according to the caloric theory, which was later shown to be false. So it has often been said that Carnot’s logic was wrong. But his logic was quite correct. Only Clapeyron’s simplified version, that everybody read, was incorrect.
The so-called second law of thermodynamics was thus discovered by Carnot before the first law! It would be interesting to give Carnot’s argument that did not use the first law, but we shall not do so because we want to learn physics, not history. We shall use the first law from the start, in spite of the fact that a great deal can be done without it.
Let us begin by stating the first law, the conservation of energy: if one has a system and puts heat into it, and does work on it, then its energy is increased by the heat put in and the work done. We can write this as follows: The heat Q put into the system, plus the work W done on the system, is the increase in the energy U of the system; the latter energy is sometimes called the internal energy:
Change in U=Q+W. (44.1)
The change in U can be represented as adding a little heat ΔQ and adding a little work ΔW:
ΔU=ΔQ+ΔW, (44.2)
which is a differential form of the same law.
 الاكثر قراءة في الديناميكا الحرارية
الاكثر قراءة في الديناميكا الحرارية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















