


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-8-2017
Date: 6-4-2016
Date: 20-12-2015
|
CHLORINATION OF TOLUENE
The chlorination of toluene by substituting the methyl hydrogens is a free radical reaction. A mixture of three chlorides (benzyl chloride, benzal chloride and benzotrichloride) results.
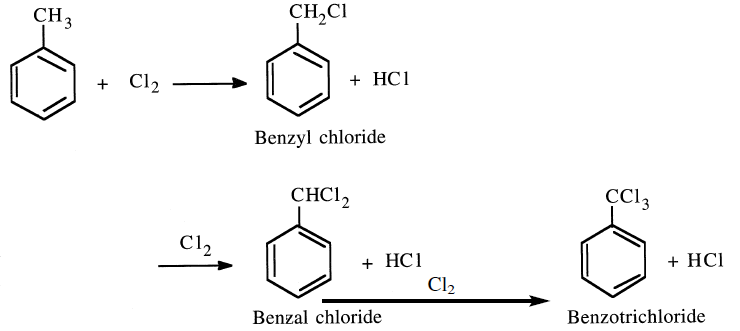
The ratio of the chloride mixture mainly derives from the toluene/chlorine ratio and the contact time. Benzyl chloride is produced by passing dry chlorine into boiling toluene (110°C) until reaching a density of 1.283. At this density, the concentration of benzyl chloride reaches the maximum. Light can initiate the reaction. Benzyl chloride can produce benzyl alcohol by hydrolysis:
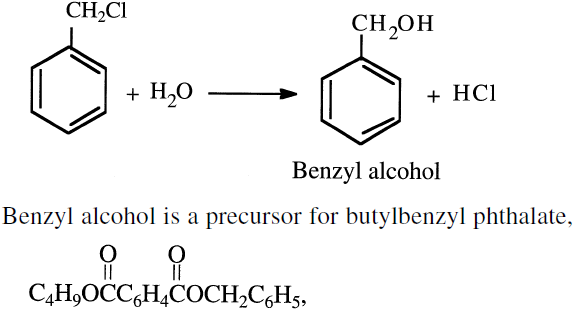
a vinyl chloride plasticizer. Benzyl chloride is also a precursor for phenylacetic acid via the intermediate benzyl cyanide. Phenylacetic acid is used to make phenobarbital (a sedative) and penicillin G. Benzal chloride is hydrolyzed to benzaldehyde, and benzotrichloride is hydrolyzed to benzoic acid. Chlorinated toluenes are not large-volume chemicals, but they are precursors for many synthetic chemicals and pharmaceuticals.



|
|
|
|
مخاطر عدم علاج ارتفاع ضغط الدم
|
|
|
|
|
|
|
اختراق جديد في علاج سرطان البروستات العدواني
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|