


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-5-2016
Date: 11-9-2017
Date: 3-9-2017
|
Caprolactam Production
Caprolactam, a white solid that melts at 69°C, can be obtained either in a fused or flaked form. It is soluble in water, ligroin, and chlorinated hydrocarbons. Caprolactam’s main use is to produce nylon 6. Other minor uses are as a crosslinking agent for polyurethanes, in the plasticizer industry, and in the synthesis of lysine.
The first step in producing caprolactam from benzoic acid is its hydrogenation to cyclohexane carboxylic acid at approximately 170°C and 16 atmospheres over a palladium catalyst:
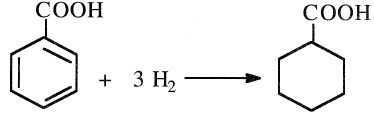
The resulting acid is then converted to caprolactam through a reaction with nitrosyl-sulfuric acid:
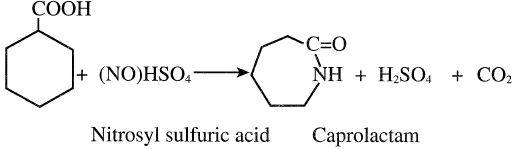
Figure 1.1 shows an integrated caprolactam production process. Toluene, the feed, is first oxidized to benzoic acid. Benzoic acid is then hydrogenated to cyclohexane carboxylic acid, which reacts with nitrosylsulfuric acid yielding caprolactam. Nitrosyl sulfuric acid comes from reacting nitrogen oxides with oleum. Caprolactam comes as an acidic solution that is neutralized with ammonia and gives ammonium sulfate as a by-product of commercial value. Recovered caprolactam is purified through solvent extraction and fractionation.
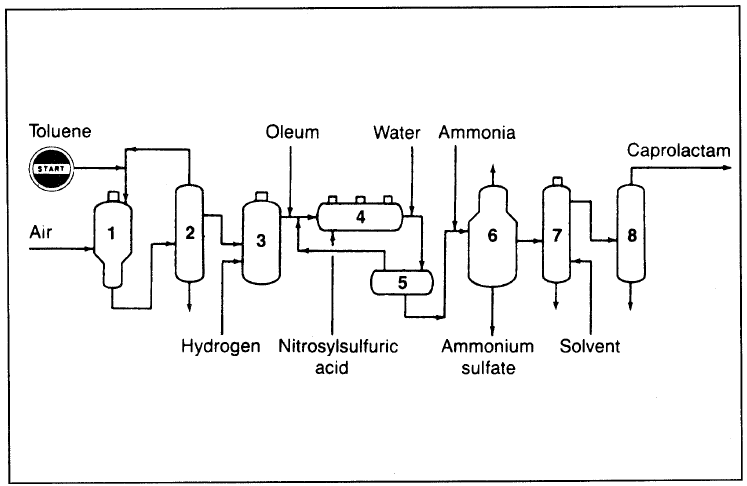
Figure 1.1. The SNIA BPD process for producing caprolactam: (1) toluene oxidation reactor, (2) fractionator, (3) hydrogenation reactor (stirred autoclave), (4) multistage reactor (conversion to caprolactam), (5) water dilution, (6) crystallizer, (7) solvent extraction, (8) fractionator.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|