


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-7-2017
Date: 23-7-2017
Date: 22-8-2017
|
Benzaldehyde
Oxidizing toluene to benzaldehyde is a catalyzed reaction in which a selective catalyst limits further oxidation to benzoic acid. In the first step, benzyl alcohol is formed and then oxidized to benzaldehyde. Further oxidation produces benzoic acid:
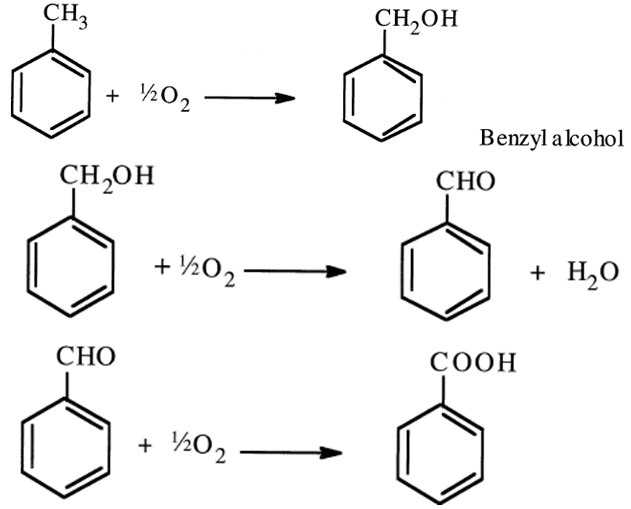
The problem with this reaction is that each successive oxidation occurs more readily than the preceding one (more acidic hydrogens after introducing the oxygen hetero atom, which facilitates the oxidation reaction to occur). In addition to using a selective catalyst, the reaction can be limited to the production of the aldehyde by employing short residence times and a high toluene-to-oxygen ratio. In one process, a mixture of UO2 (93%) and MnO2 (7%) is the catalyst. Ayield of 30–50% could be obtained at low conversions of 10–20%. The reaction temperature is approximately 500°C.
In another process, the reaction goes forward in the presence of methanol over an FeBr2—CoBr2 catalyst mixture at approximately 100–140°C.
Benzaldehyde has limited uses as a chemical intermediate. It is used as a solvent for oils, resins, cellulose esters, and ethers. It is also used in flavoring compounds and in synthetic perfumes.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
مكتب السيد السيستاني يعزي أهالي الأحساء بوفاة العلامة الشيخ جواد الدندن
|
|
|