


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-9-2017
Date: 18-9-2017
Date: 5-9-2017
|
Aromatics Production
Liquefied petroleum gas (LPG), a mixture of propane and butanes, is catalytically reacted to produce an aromatic-rich product. The first step is assumed to be the dehydrogenation of propane and butane to the corresponding olefins followed by oligomerization to C6, C7, and C8 olefins. These compounds then dehydrocyclize to BTX aromatics. The following reaction sequence illustrates the formation of benzene from 2 propane molecules:
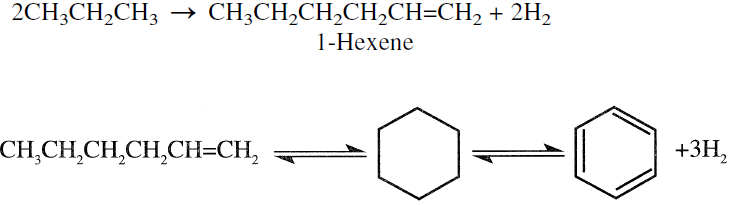
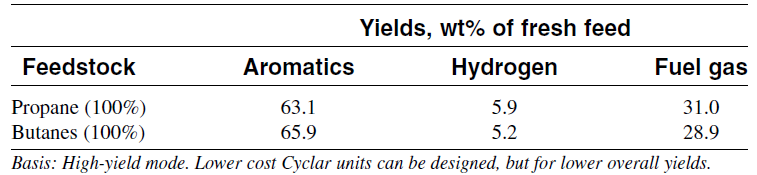
Although olefins are intermediates in this reaction, the final product contains a very low olefin concentration. The overall reaction is endothermic due to the predominance of dehydrogenation and cracking. Methane and ethane are by-products from the cracking reaction. Table 1.1 shows the product yields obtained from the Cyclar process developed jointly by British Petroleum and UOP.
Table 1.1: Product yield from saturated LPG feed to the cyclar process
A simplified flow scheme for the Cyclar process is shown in Figure 1.1.
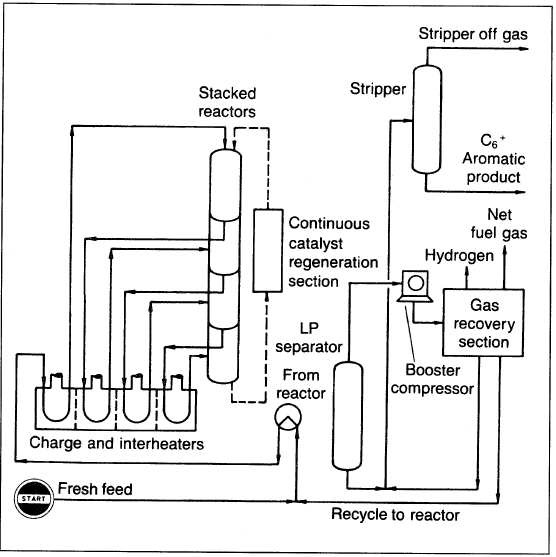
Figure 1.1. A flow diagram showing the Cyclar process for aromatization of LPG.
The process consists of a reactor section, continuous catalyst regeneration unit (CCR), and product recovery section. Stacked radial-flow reactors are used to minimize pressure drop and to facilitate catalyst recirculation to and from the CCR. The reactor feed consists solely of LPG plus the recycle of unconverted feed components; no hydrogen is recycled. The liquid product contains about 92 wt% benzene, toluene, and xylenes (BTX) (Figure 1.2), with a balance of C9+ aromatics and a low nonaromatic content. Therefore, the product could be used directly for the recovery of benzene by fractional distillation (without the extraction step needed in catalytic reforming).
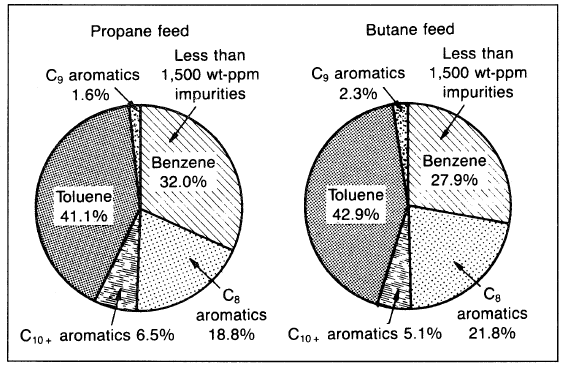
Figure 1.2. The liquid (C6+) product breakdown in weight units obtained from the Cyclar process.
Interest in the use of lower-value light paraffins for the production of aromatics led to the introduction of two new processes similar to the Cyclar process, the Z-forming and the Aroformer processes, which were developed in Japan and Australia, respectively.
Research is also being conducted in Japan to aromatize propane in presence of carbon dioxide using a Zn-loaded HZSM-5 catalyst. The effect of CO2 is thought to improve the equilibrium formation of aromatics by the consumption of product hydrogen (from dehydrogenation of propane) through the reverse water gas shift reaction.
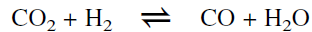
However, it was found that the effect on the equilibrium formation of aromatics is not substantial due to thermodynamic considerations. A more favorable effect was found for the reaction between ethylene (formed via cracking during aromatization of propane) and hydrogen. The reverse shift reaction consumes hydrogen and decreases the chances for the reduction of ethylene to ethane byproduct.
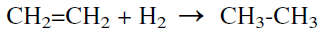



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|