

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Cationic Polymerization
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p 306
18-9-2017
2063
Cationic Polymerization
Strong protonic acids can affect the polymerization of olefins. Lewis acids, such as AlCl3 or BF3, can also initiate polymerization. In this case, a trace amount of a proton donor (cocatalyst), such as water or methanol, is normally required. For example, water combined with BF3 forms a complex that provides the protons for the polymerization reaction.
An important difference between free radical and ionic polymerization is that a counter ion only appears in the latter case. For example, the intermediate formed from the initiation of propene with BF3-H2O could be represented as
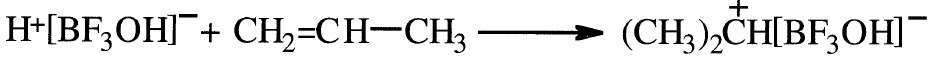
The next step is the insertion of the monomer molecules between the ion pair.
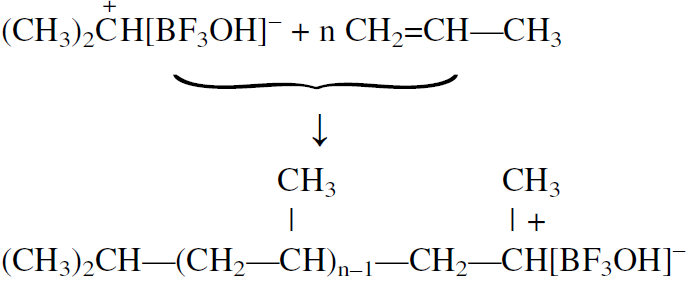
In ionic polymerizations, reaction rates are faster in solvents with high dielectric constants, which promote the separation of the ion pair.
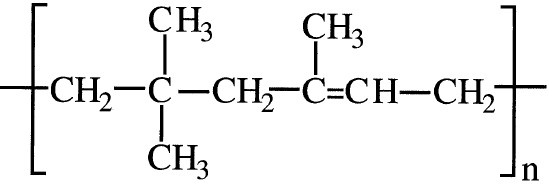
Cationic polymerizations work better when the monomers possess an electron-donating group that stabilizes the intermediate carbocation. For example, isobutylene produces a stable carbocation, and usually copolymerizes with a small amount of isoprene using cationic initiators. The product polymer is a synthetic rubber widely used for tire inner tubes:
Cationic initiators can also polymerize aldehydes. For example, BF3 helps produce commercial polymers of formaldehyde. The resulting polymer, a polyacetal, is an important thermoplastic:
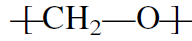
In general, the activation energies for both cationic and anionic polymerization are small. For this reason, low-temperature conditions are normally used to reduce side reactions. Low temperatures also minimize chain transfer reactions. These reactions produce low-molecular weight polymers by disproportionation of the propagating polymer:
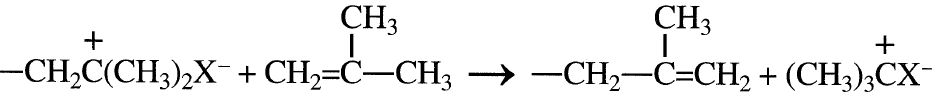
X– represents the counter ion. Cationic polymerization can terminate by adding a hydroxy compound such as water:
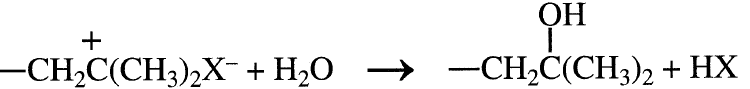
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















