


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-10-2018
Date: 22-10-2018
Date: 23-2-2019
|
Binary hydrides The group 14 elements
Silane, SiH4, is formed when SiCl4 or SiF4 reacts with Li[AlH4] and is a source of pure Si (equation 1.1) for semiconductors. Silanes SinH2n+2 with straight or branched chains are known for 1 ≤ n ≤ 10, and Figure 1.1 compares the boiling points of the first five straight-chain silanes with their hydrocarbon analogues. Silanes are explosively inflammable in air (equation 1.2).
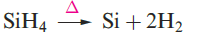 (1.1)
(1.1)
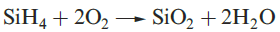 (1.2)
(1.2)
A mixture of SiH4, Si2H6, Si3H8 and Si4H10 along with traces of higher silanes is obtained when Mg2Si reacts with aqueous acid, but the non-specificity of this synthesis renders it of little practical value. By irradiating SiH4 with a CO2 laser, SiH4 can be converted selectively into Si2H6. Silane is a colourless gas which is insoluble in water, reacts rapidly with alkalis (equation 1.3) and forms compounds of the type M[SiH3] with Na, K (equation 1.4), Rb and Cs.The crystalline salt K[SiH3] possesses an NaCl structure and is a valuable synthetic reagent, e.g. equation 1.5.
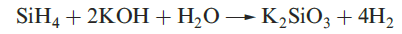 (1.3)
(1.3)
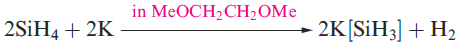 (1.4)
(1.4)
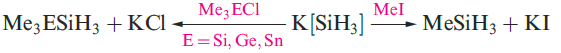 (1.5)
(1.5)
Germanes GenH2n+2 (straight and branched chain isomers) are known for 1 ≤ n ≤ 9. GeH4 is less reactive than SiH4; it is a colourless gas (bp 184 K, dec 488 K), insoluble in water, and prepared by treating GeO2 with Na[BH4] although higher germanes are also formed. Discharges of various frequencies are finding increased use for this type of synthesis and have been used to convert GeH4 into higher germanes, or mixtures of SiH4 and GeH4 into Ge2H6, GeSiH6 and Si2H6. Mixed hydrides of Si and Ge, e.g. GeSiH6 and GeSi2H8, are also formed when an intimate mixture of Mg2Ge and Mg2Si is treated with acid. Reactions between GeH4 and alkali metals, M, in liquid NH3 produce M[GeH3], and, like [SiH3]-, the [GeH3]- ion is synthetically useful. The reaction of SnCl4 with Li[AlH4] gives SnH4 (bp 221 K) but this decomposes at 298K into Sn and H2; note the variation in reactivities: SiH4 > GeH4 < SnH4. Plumbane, PbH4, is poorly characterized and may not actually have been isolated. Significantly, however, replacement of H atoms by alkyl or aryl substituents is accompanied by increased stability.
Silanes SinH2n+2 with straight or branched chains are known for 1 ≤ n ≤ 10, and Figure 1.1 compares the boiling points of the first five straight-chain silanes with their hydrocarbon analogues. Silanes are explosively inflammable in air (equation1.2).
A mixture of SiH4, Si2H6, Si3H8 and Si4H10 along with traces of higher silanes is obtained when Mg2Si reacts with aqueous acid, but the non-specificity of this synthesis renders it of little practical value. By irradiating SiH4 with a CO2 laser,SiH4 can be converted selectively into Si2H6.
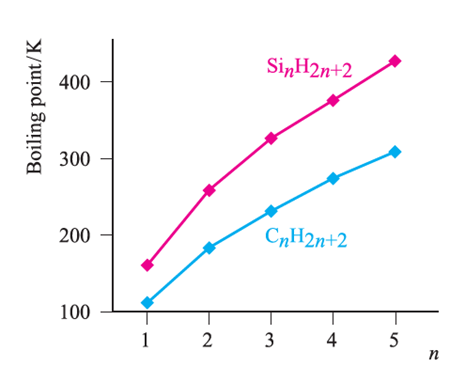
Fig. 1.1 Boiling points of the straight-chain silanes, SinH2n+2, and hydrocarbons CnH2n+2.
Silane is a colourless gas which is insoluble in water, reacts rapidly with alkalis (equation 1.3) and forms compounds of the type M[SiH3] with Na, K (equation 1.4), Rb and Cs. The crystalline salt K[SiH3] possesses an NaCl structure and is a valuable synthetic reagent, e.g. equation 1.5.
Germanes GenH2n+2 (straight and branched chain isomers) are known for 1 ≤ n ≤ 9. GeH4 is less reactive than SiH4; it is a colourless gas (bp 184 K, dec 488 K), insoluble in water, and prepared by treating GeO2 with Na[BH4] although higher germanes are also formed. Discharges of various frequencies are finding increased use for this type of synthesis and have been used to convert GeH4 into higher germanes, or mixtures of SiH4 and GeH4 into Ge2H6, GeSiH6 and Si2H6. Mixed hydrides of Si and Ge, e.g. GeSiH6 and GeSi2H8, are also formed when an intimate mixture of Mg2Ge and Mg2Si is treated with acid.
Reactions between GeH4 and alkali metals, M, in liquid NH3 produce M[GeH3], and, like [SiH3]-, the [GeH3]- ion is synthetically useful. The reaction of SnCl4 with Li[AlH4] gives SnH4 (bp 221 K) but this decomposes at 298K into Sn and H2; note the variation in reactivities: SiH4 > GeH4 < SnH4. Plumbane, PbH4, is poorly characterized and may not actually have been isolated. Significantly, however, replacement of H atoms by alkyl or aryl substituents is accompanied by increased stability.
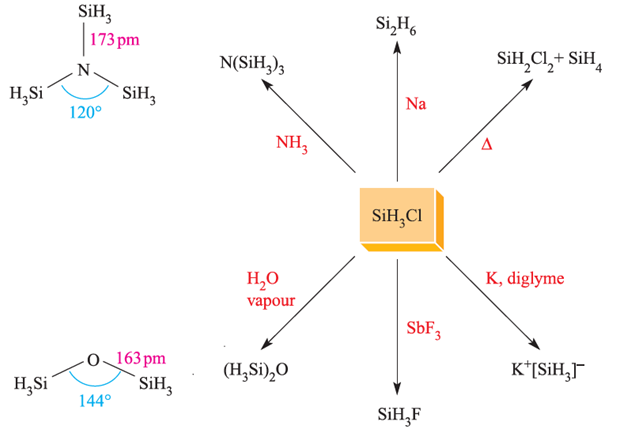
Fig. 1.2 Representative reactions of SiH3Cl. The structures of N(SiH3)3 (determined by Xray diffraction at 115 K) and (H3Si)2O (determined by electron diffraction).



|
|
|
|
مخاطر عدم علاج ارتفاع ضغط الدم
|
|
|
|
|
|
|
اختراق جديد في علاج سرطان البروستات العدواني
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|