


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-8-2016
Date: 20-6-2019
Date: 18-6-2019
|
Isomerism in d-block metal complexes
In this book so far, we have not mentioned isomerism very often, and most references have been to trans- and cis-isomers, e.g. trans-[CaI2)THF(4] and the trans- and cis-isomers of N2F2. These are geometrical isomers, and our previous discussion of this topic will not be elaborated further here.
Self-study exercises
1. Draw possible structures for the square planar complexes [PtBr2(py)2] and [PtCl3(PEt3)]-and give names to distinguish between any isomers that you have drawn.
2. In [Ru(CO)4(PPh3)], the Ru centre is in a trigonal bipyramidal environment. Draw the structures of possible isomers and give names to distinguish between them.
3. Draw the structures and name the isomers of octahedral [CrCl2(NH3)4]+.
4. Octahedral [RhCl3(H2O)3] has two isomers. Draw their structures and give them distinguishing names.
In this section, we shall be concerned with other types of isomerism exhibited by d-block metal complexes, and we use a classification that goes back to the work of Werner (Figure 1.1).
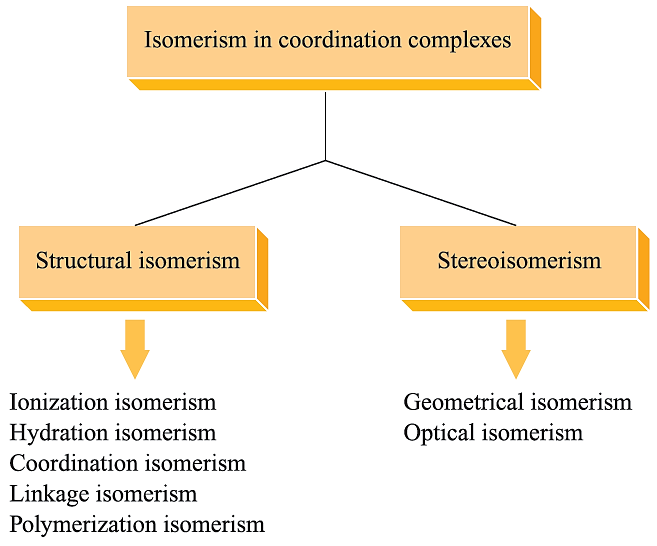
Fig. 1.1 Classification of types of isomerism in metal complexes.



|
|
|
|
الصين.. طريقة لمنع تطور قصر النظر لدى تلاميذ المدارس
|
|
|
|
|
|
|
ماذا سيحدث خلال كسوف الشمس يوم السبت؟
|
|
|
|
|
|
|
المجمع العلمي يختتم الختمات القرآنية في مقام الإمام المهدي (عجّل الله فرجه)
|
|
|