


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-2-2017
Date: 23-2-2017
Date: 22-2-2017
|
Applying VB theory
We illustrate the applications and limitations of VB theory by considering octahedral complexes of Cr(III) (d3) and Fe(III) (d5) and octahedral, tetrahedral and square planar complexes of Ni(II) (d8). The atomic orbitals required for hybridization in an octahedral complex are the 3dz2 , 3dx2-y2, 4s, 4px, 4py and 4pz (Table 20.1); these orbitals must be unoccupied so as to be available to accept six pairs of electrons from the ligands. The Cr3+ ion has three unpaired electrons and these are accommodated in the 3dxy, 3dxz and 3dyz orbitals:
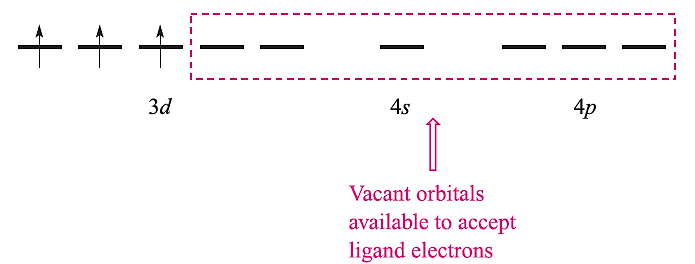
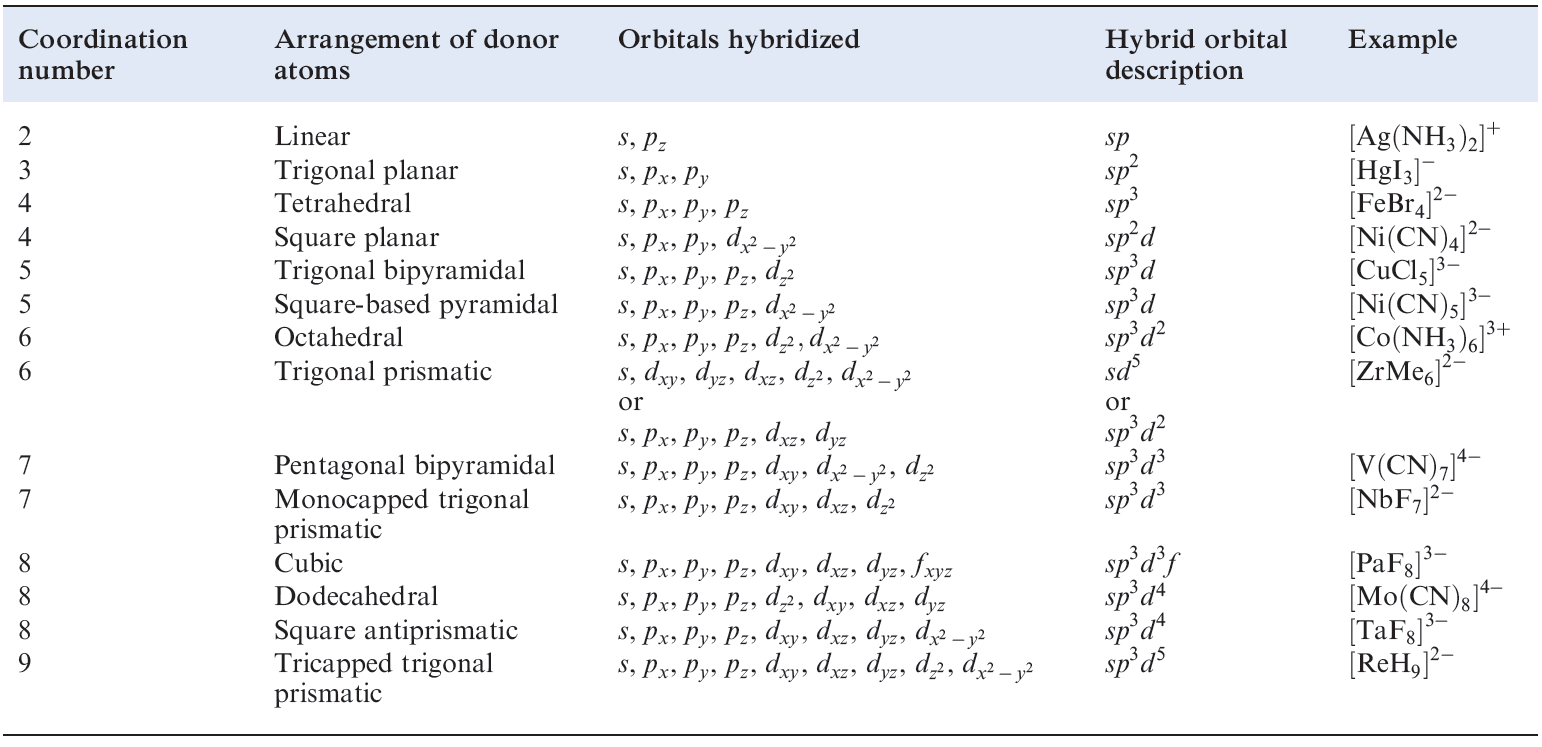
Table 1.1 Hybridization schemes for the σ-bonding frameworks of different geometrical configurations of ligand donor atoms.
With the electrons from the ligands included and a hybridization scheme applied for an octahedral complex, the diagram becomes:
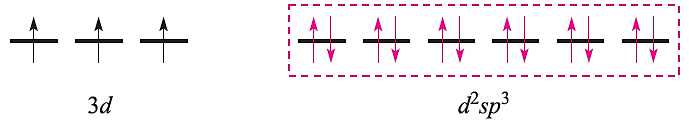
This diagram is appropriate for all octahedral Cr(III) complexes because the three 3d electrons always singly occupy different orbitals. For octahedral Fe(III) complexes, we must account for the existence of both high- and low-spin complexes. The electronic configuration of the free Fe3+ ion is:
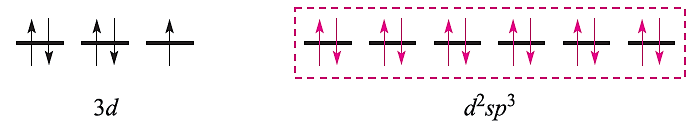
For a high-spin octahedral complex such as [FeF6]3-, the five 3d electrons occupy the five 3d atomic orbitals (as in the free ion shown above) and the two d orbitals required for the sp3d2 hybridization scheme must come from the 4d set. With the ligand electrons included, valence bond theory describes the bonding as follows leaving three empty 4d atomic orbitals (not shown):
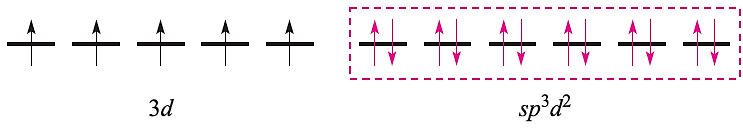
This scheme, however, is unrealistic because the 4d orbitals are at a significantly higher energy than the 3d atomic orbitals. Nickel(II) (d8) forms paramagnetic tetrahedral and octahedral complexes, and diamagnetic square planar complexes. Bonding in a tetrahedral complex can be represented as follows (ligand electrons are shown in red):
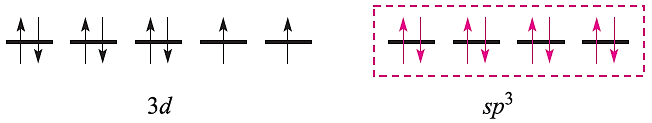
and an octahedral complex can be described by the diagram:
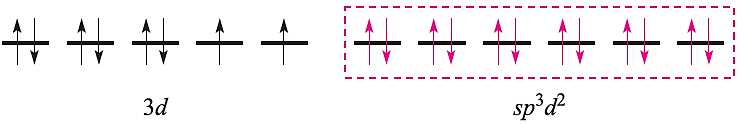
in which the three empty 4d atomic orbitals are not shown. For diamagnetic square planar complexes, valence bond theory gives the following picture:
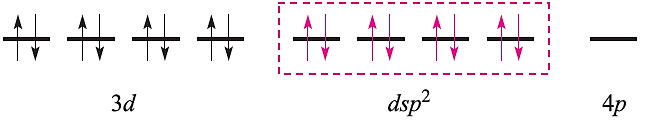
Valence bond theory may rationalize stereochemical and magnetic properties, but only at a simplistic level. It can say nothing about electronic spectroscopic properties or about the kinetic inertness that is a characteristic of the low-spin d6 configuration. Furthermore, the model implies a distinction between high- and low-spin complexes that is actually misleading. Finally, it cannot tell us why certain ligands are associated with the formation of high- (or low-)spin complexes. We therefore move on to alternative approaches to the bonding.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|