


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-2-2017
Date: 7-7-2020
Date: 25-6-2020
|
Dealing with multiple bonds
I define covalent bonding as the sharing of one or more electron pairs. In hydrogen and most other diatomic molecules, only one electron pair is shared. But in many covalent bonding situations, the atoms share more than one electron pair.
For instance, nitrogen (N2) is a diatomic molecule in which the atoms share more than one electron pair. The nitrogen atom is in the VA family on the periodic table, meaning that it has five valence electrons .
So nitrogen needs three more valence electrons to complete its octet. A nitrogen atom can fill its octet by sharing three electrons with another nitrogen atom, forming three covalent bonds, a so-called triple bond. Figure 1-1 shows the triple bond formation of nitrogen.
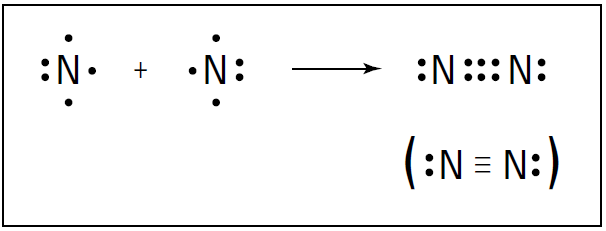
Figure 1-1: Triple bond formation in N2.
A triple bond isn’t quite three times as strong as a single bond, but it’s a very strong bond. In fact, the triple bond in nitrogen is one of the strongest bonds known. This strong bond is what makes nitrogen very stable and resistant to reaction with other chemicals. It’s also why many explosive compounds (such as TNT and ammonium nitrate) contain nitrogen: When these compounds break apart in a chemical reaction, nitrogen gas (N2) is formed, and a large amount of energy is released. Carbon dioxide (CO2) is another example of a compound containing a multiple bond. Carbon can react with oxygen to form carbon dioxide. Carbon has four valence electrons, and oxygen has six. Carbon can share two of its valence electrons with each of the two oxygen atoms, forming two double bonds. Figure 1-2 shows these double bonds.
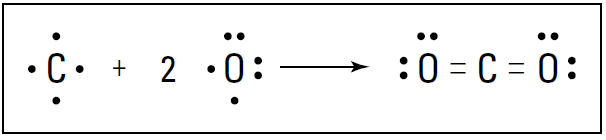
Figure 1-2: Formation of carbon dioxide.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|