


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 20-6-2019
Date: 20-6-2019
Date: 23-2-2017
|
High- and low-spin states
We consider magnetic properties in detail, but for now, let us simply state that magnetic data allow us to determine the number of unpaired electrons. In an isolated first row d-block metal ion, the 3d orbitals are degenerate and the electrons occupy them according to Hund’s rules: e.g. diagram 20.1 shows the arrangement of six electrons.
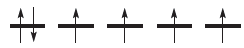
However, magnetic data for a range of octahedral d6 complexes show that they fall into two categories: paramagnetic or diamagnetic. The former are called high-spin complexes and correspond to those in which, despite the d orbitals being split, there are still four unpaired electrons.
The diamagnetic d6 complexes are termed low-spin and correspond to those in which electrons are doubly occupying three orbitals, leaving two unoccupied.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
مكتب السيد السيستاني يعزي أهالي الأحساء بوفاة العلامة الشيخ جواد الدندن
|
|
|