


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-12-2015
Date: 31-3-2021
Date: 18-3-2021
|
Electron Paramagnetic Resonance
1. Introduction to Electron Paramagnetic Resonance (EPR) Spectroscopy
Electron paramagnetic resonance (EPR) refers to spectroscopy of unpaired electrons, and some aspects of the technique are termed “electron spin resonance” (ESR) or “electron magnetic resonance” (EMR). Unpaired electrons in biological systems are in much lower abundance than nuclei; thus, EPR is a technique that focuses on local sites, whereas nuclear magnetic resonance (NMR) tends to be more global. A connection exists between EPR and NMR in the variations of EPR that are termed “electron spin echo envelope modulation” (ESEEM) and “electron nuclear double resonance” (ENDOR). In these techniques, the energies of the transitions involving electrons are modulated by nearby nuclei, and this effect provides information about the type and geometry of nuclei around the site of an unpaired electron. The term “spin” in the designations of various forms of magnetic resonance spectroscopy refers to a property of electrons (or nuclei), and it is the interaction of the spin with the magnetic field that leads to separation of energy levels between which the spectroscopic transition occurs. A magnetic field is usually required for EPR, as is a source of energy to effect transitions. In addition, a few cases exist where spin transitions can be detected in the absence of a magnetic field.
Electron transfer systems (for instance, those in photosynthetic membranes) provide excellent opportunities to follow the intimate details of the transfer process with EPR spectroscopy. Other naturally occurring sources of unpaired electrons that are subjects for this form of spectroscopy include complexes of a variety of molecules with nitric oxide, quinone and flavin cofactors in enzymes, free radical enzyme intermediates, and metal ion sites in proteins. By definition, a free radical has a homolytically broken bond with an unpaired electron. The range of applications of EPR spectroscopy is not limited to these natural sources of unpaired electrons. The EPR probe technique of spin labeling is being applied to examine dynamics of DNA and proteins, to study protein folding, and to unravel the complex motions of membrane components.
Molecular biological approaches extend the applications of spin labeling, and these applications are generically referred to as site directed spin labeling. In addition, unpaired electrons in free radical intermediates that have a short lifetime can be studied by trapping them to give a longer-lived, secondary radical in an EPR-related technique called “spin trapping.” The text below will give examples of the type of information that can be obtained by applying EPR spectroscopy to these instances of unpaired electrons in biology.
2. EPR in Photosynthesis
One of the long-standing puzzles of electron transfer in photosynthetic membranes is that the photoreaction center has pairs of molecular components arranged apparently symmetrically about a plane perpendicular to the membrane surface, yet electron transfer among the components follows a path involving primarily one-half of the dimer (1). EPR studies, especially of oriented crystals of reaction centers (2), provide precise information about small differences in symmetry of the two halves of the system. From EPR studies, it is possible to determine the orientation of orbitals involved in electron transfer between cofactors, and the positions of light atoms, such as hydrogen, on the cofactors can be determined by ENDOR with great precision (1). The various components of the electron transfer pathway—chlorophylls and quinones—can be selected for EPR by optical experiments, selective depletion of some components, and molecular biology. High-frequency EPR (1) helps to separate the spectra of different sites of unpaired electrons, much as higher frequency NMR gives better resolution of different nuclei. The manner in which electrons flow ultimately into the photosynthetic oxygen-evolving complex, which contains varied numbers of unpaired electrons associated with a cluster of four manganese atoms, is another challenge. ESEEM and ENDOR studies, together with isotope labeling (3) provide details of the structure of this site.
3. EPR of Nitric Oxide Complexes
Molecular adducts of nitric oxide give a diverse set of EPR signals that can be employed to determine the pathway of nitric oxide in tissues. Ferrous iron may or may not have unpaired spins, depending on whether it is low or high spin, but, in either case, it is, respectively, an impossible or difficult subject for EPR spectroscopy. Fortunately, the adduct of nitric oxide with ferrous iron in heme is quite suitable for EPR spectroscopy and provides a characteristic signature, shown in Figure 1. This spectrum is distinct from those of the nitric oxide adduct of either amino- or thiol- groups of protein side chains, as well as from copper adducts and adducts with other forms of iron. For further delineation of the location of nitric oxide in tissues, “spin traps” for nitric oxide are available. Typical spin traps for nitric oxide are ferrous ion chelated with N-methyl-D-glucamine dithiocarbamate or with diethylthiocarbamate.

Figure 1. The EPR spectrum of deoxymyoglobin to which nitric oxide (NO) gas was added. The spectrum was recorded at 77K and a frequency of 9.1 GHz.
4. EPR of Free-Radical Protein Intermediates
Ribonucleotide reductases use metals and free radical chemistry to carry out reduction of ribonucleotides to deoxyribonucleotides. (56) In different sources of the enzyme, the presence of free-radical intermediates has been demonstrated to involve side chains of the amino acids tyrosine, tryptophan, cysteine and glycine. EPR spectroscopy of tyrosine radicals has been used in this system, as well as in photosynthetic membranes, to demonstrate that the unpaired electron density at the various carbon atoms of the tyrosine aromatic ring is fine tuned by hydrogen bonds to the phenolic oxygen and by the protein environment. Figure 2 gives examples of EPR of tyrosine radicals from ribonuclotide reductases of several sources. The process by which involvement of metal ions and radical side chains lead to a free radical intermediate of the ribonucleotide is thought to involve long-range electron transfer. Organic cofactors, such as flavins, also form intermediates that can be detected in EPR studies of enzyme reactions.

Figure 2. EPR spectra of tyrosine freeradicals of ribonucleotide reductase R2 subunits from different organisms. The EPR spectra were recorded at temperatures of 20 to 30K at ~9 GHz. The numbers given on the structure are the spin density distributions for the E. coli and S. typhimurium enzymes.
5. EPR of Metal Ions in Biology
All metals ions containing one or more unpaired electrons (paramagnetic ions) are in principle subjects for study by EPR, but the conditions for the experiments are quite varied (7). The paramagnetic ions in proteins that are commonly studied include manganese 2+, copper 2+, iron 3, +occasionally iron 2+, nickel3+, and cobalt2+. The conditions for the spectroscopy differ drastically, depending on which metal is the subject of study. Sometimes, mangenese ions can be detected with high sensitivity in solution at room temperature, but detection of 17O ligands to the Mn2+ (for example from a threonine side chain in p21 ras) required both low temperature and high frequency EPR (8). ENDOR and ESEEM (1) are alternative methods for detecting nuclei, such as nitrogen or hydrogen bound to, or near, metal-ion sites. Copper sites also can be detected at room temperature, but sensitivity is improved by conducting the EPR experiments at or lower than liquid nitrogen temperature. Studies of iron are almost always done at a temperature near that of liquid helium. The two primary reasons that low temperature is used in metal ion EPR are that relaxation times are too fast for temperature studies, and the signal intensity increases inversely with temperature.
Quantitative evaluation of the number of unpaired spins in different sites within a complex biochemical electron transfer system can be made by EPR and associated studies. For experiments of this type, calculations of the theoretical spectra are often needed for interpretation. Recent advances in EPR spectroscopy, particularly high-frequency EPR, provide improved resolution in samples with multiple EPR-detectable sites. Figure 3 shows a high frequency EPR spectrum of copper ion in lactoferrin, in which the copper spectrum is well separated from the signal of a manganese impurity (8, 9) . At lower EPR frequencies, the manganese signal is superimposed on the central portion of the copper signal.
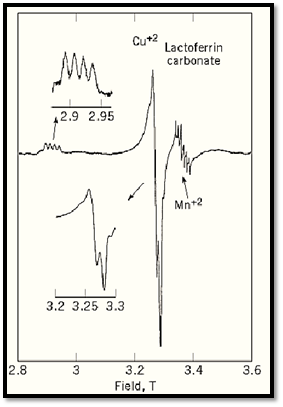
Figure 3. High-frequency EPR spectrum of di-cupric lactoferrin. The spectrum was recorded at 40 K and 94.1 GHz. The two regions from which significant information can be obtained are amplified in insets on the left. A feature indicated on the right is the EPR signal from an impurity of manganese ion in the sample.
6. Spin Trapping and EPR Imaging
The EPR spectrum shown in Figure 1 was actually from a spin trapping experiment (4). In that case, the effluent from stimulated endothelial cells was passed into a solution of deoxy myoglobin, and the nitric oxide was trapped as the myoglobin heme adduct. Spin traps can also exhibit therapeutic effects in inhibition of lipoprotein oxidation (10). In a sense, EPR imaging of oxygen concentration in tissues of animals is also a spin trap experiment, but, in this case, only the magnetism of oxygen is “trapped” through its magnetic, but not chemical, interactions with a spin label. The degree of broadening of the EPR signal is transformed into an image (11).
References
1. H. Levanon and K. Möbius (1997) Ann. Rev. Biophys. Biomol. Struct. 26, 495–540.
2. W. Hofbauer, A. Zouni, R. Bittl, J. Kern, P. Orth, F. Lendzian, P. Fromme, H. T. Witt, and W. Lubitz (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6623–2228.
3. R. D. Britt, J. M. Peloquin, and K. A. Campbell (2000) Ann. Rev. Biophys. Biomol. Struct. 29, 463-495.
4. D. J. Singel and J. R. Lancaster, Jr. (1996) Methods in Nitric Oxide Research, M. Feelisch and J. S. Stamler, eds. John Wiley & Sons, pp. 341–356.
5. J. A. Stubbe (2001) Trends Biochem. Sci. 26, 93–99.
6. A. Gräslund and M. Sahlin (1996) Ann. Rev. Biophys. Biomol. Struct. 27, 259–286.
7. L. J. Berliner and J. Reuben, eds. (1993) In Biological Magnetic Resonance, Vol. 13, EPR of paramagnetic molecules. Plenum, New York.
8. B. F. Bellew, C. J. Halkides, G. G. Gerfen, R. G. Griffin, and D. J. Singel (1996) Biochem 35, 12186-12193.
9. B. J. Gaffney, B. C. Maguire, R. T. Weber, and G. G. Maresch (1999) Appl. Magn. Res., 16, 207-222.
10. C. E. Thomas, D. F. Ohlweiler, and B. Kalyanaraman (1994) J. Biol. Chem. 269, 28055–28061.
11. A. T. Yordanov, K. Yamada, M. C. Krishna, J. B. Mitchell, E. Woller, M. Cloninger, and M. W. Brechbiel (2001) Angew. Chem. Int. Ed. 40, 2690–2691.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العبّاسية المقدّسة تدعو جامعة واسط للمشاركة في الحفل المركزيّ لتخرّج طلبة الجامعات
|
|
|