


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 20-3-2016
Date: 7-3-2016
Date: 8-11-2020
|
EINSTEIN AND PLANCK: E = hv
Once again we reiterate the importance of Planck’s quantization approach. Planck’s constant was originally devised to explain the spectra produced by blackbody radiators. It was actually Einstein who introduced the concept of the photon, based primarily on theory (he was unarguably the greatest theoretical physicist of all time). Photons can be thought of as literally a little packet of light and have energy proportional to their frequency and hence inversely proportional to their wavelength. The mathematical expression of this energy is
E = hv (1.1)
where n is the frequency in hertz and h is Planck’s constant. Substituting wavelength for frequency, we find that
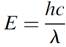
where c is the speed of light and λ the wavelength in meters. Returning to the line spectra of an atom, if one observes the wavelength of a line, the corresponding energy of the emitted photon in joules, or more conveniently in eV, with 1 eV = 1.602 × 10-19 J, may be computed using this relationship. If we now calculate the energy of 400-nm (violet) photons, we find an energy of 3.0 eV. Red photons at 700 nm have an energy of 1.8 eV. This concept of the photon has been confirmed by many experiments, including observations of the photoelectric effect, whereby incident light can cause electrons to be emitted from a metal surface in a vacuum.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تدعو جامعة الأنبار للمشاركة في الحفل المركزي لتخرج طلبة الجامعات
|
|
|