

 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 13-3-2016
Date: 17-3-2016
Date: 2-3-2016
|
The Principles of Antibiotic Therapy
Introduction
Specific antibacterial therapy refers to treatment of infections with anti- infective agents directed against the infecting pathogen. The most important group of anti-infective agents are the antibiotics, which are products of fungi and bacteria (Streptomycetes). Anti-infective agents are categorized as having a broad, narrow, or medium spectrum of action. The efficacy, or effectiveness, of a substance refers to its bactericidal or bacteriostatic effect. Anti-infective agents have many different mechanisms of action. Under the influence of sulfonamides and trimethoprim, bacteria do not synthesize sufficient amounts of tetrahydrofolic acid. All betalactam antibiotics irreversibly block the biosynthesis of murein. Rifamycin inhibits the DNA-dependent RNA polymerase (transcription). Aminoglycosides, tetracyclines, and macrolides block translation. All 4-quinolones damage cellular DNA topology by inhibiting bacterial topoisomerases. Due to their genetic variability, bacteria may develop resistance to specific anti-infective agents. The most important resistance mechanisms are: inactivating enzymes, resistant target molecules, reduced influx, increased efflux. Resistant strains (problematic bacteria) occur frequently among hospital flora, mainly Enterobacteriaceae, pseudomonads, staphylococci, and enterococci. Laboratory resistance testing is required for specific antibiotic therapy. Dilutions series tests are quantitative resistance tests used to determine the minimum inhibitory concentration (MIC). The disk test is a semiquantitative test used to classify the test bacteria as resistant or susceptible. In combination therapies it must be remembered that the interactions of two or more antibiotics can give rise to an antagonistic effect. Surgical chemoprophylaxis must be administered as a short-term antimicrobial treatment only.
Definitions
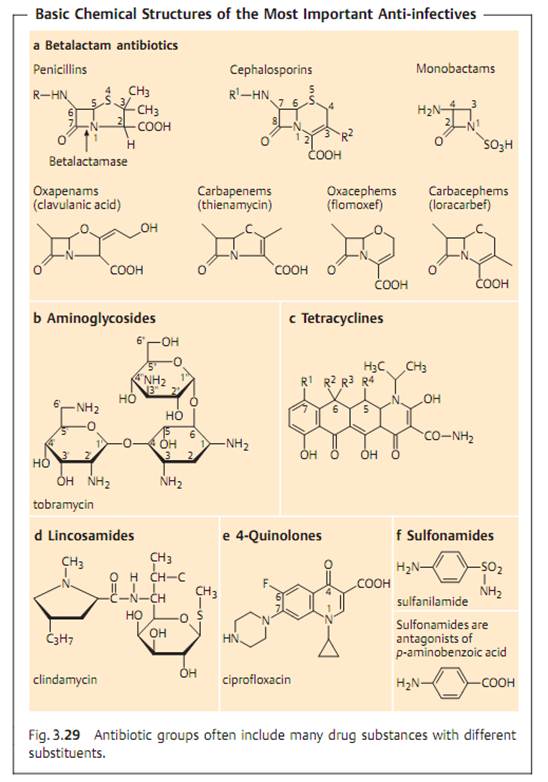
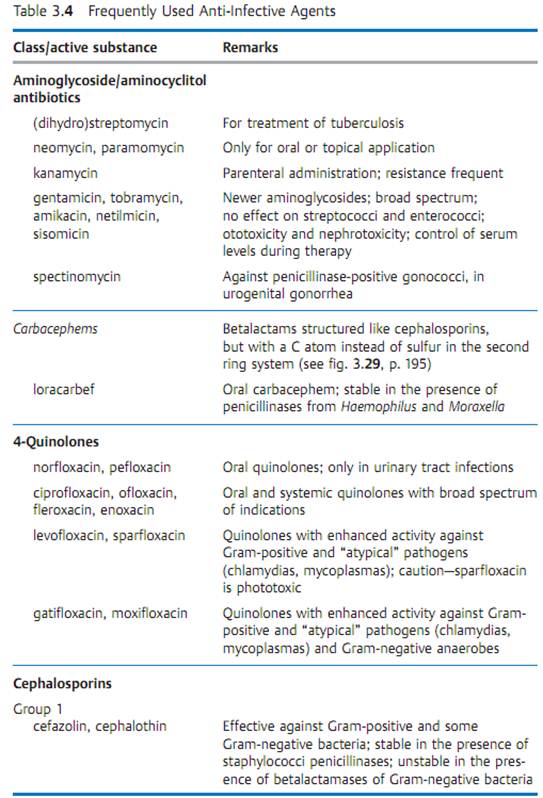
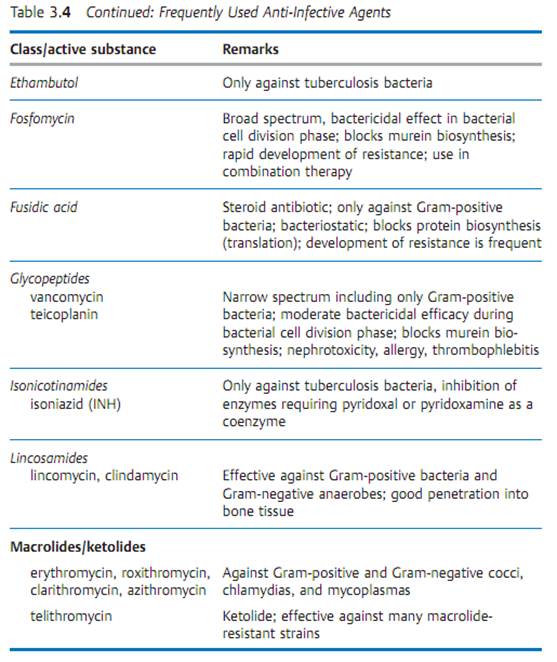
Specific antibacterial therapy designates treatment of infections with anti- infective agents directed against the infecting pathogen (syn. antibacterial chemotherapeutics, antibiotics). One feature of these pharmaceuticals is “selective toxicity,” that is, they act upon bacteria at very low concentration levels without causing damage to the macroorganism. The most important group of anti-infective agents is the antibiotics. These natural substances are produced by fungi or bacteria (usually Streptomycetes). The term “antibiotic” is often used in medical contexts to refer to all antibacterial pharmaceuticals, not just to antibiotics in this narrower sense. Fig. 3.28 illustrates the relations between an anti-infective agent, the host organism, and a bacterial pathogen. Table 3.4 lists frequently used anti-infective agents. The most important groups (cephalosporins, penicillins, 4-quinolones, macrolides, tetracyclines) are in bold print. Fig. 3.29 presents the basic chemical structures of the most important anti-infective agents.





Spectrum of Action
Each anti-infective agent has a certain spectrum of action, which is a range of bacterial species showing natural sensitivity to the substance. Some anti- infective agents have a narrow spectrum of action (e.g., vancomycin). Most, however, have broad spectra like tetracyclines, which affect all eubacteria.
Efficacy
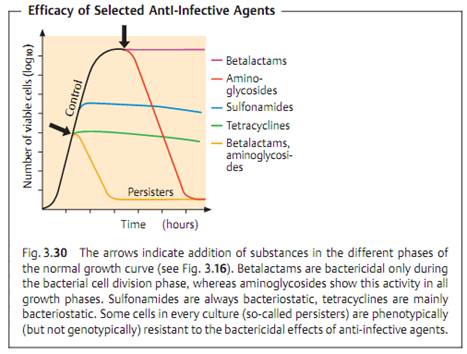
The efficacy of an anti-infective agent (syn. kinetics of action) defines the way it affects a bacterial population. Two basic effects are differentiated: bacteriostasis, i.e., reversible inhibition of growth, and irreversible bactericidal activity (Fig. 3.30). Many substances can develop both forms of efficacy depending on their concentration, the type of organism, and the growth phase. Many of these drugs also have a postantibiotic effect (PAE) reflecting the damage inflicted on a bacterial population. After the anti-infective agent is no longer present, the bacterial cells not killed require a recovery phase before they can reproduce again. The PAE may last several hours.
A bacteriostatic agent alone can never completely eliminate pathogenic bacteria from the body's tissues. “Healing” results from the combined effects of the anti-infective agent and the specific and nonspecific immune defenses of the host organism. In tissues in which this defense system is inefficient (endocardium), in the middle of a purulent lesion where no functional phagocytes are present, or in immunocompromised patients, bactericidal substances must be required. The clinical value of knowing whether an antibacterial drug is bacteriostatic or bactericidal is readily apparent.
All of the bacteria from an infection focus cannot be eliminated without support from the body's immune defense system. A bacterial population always includes several cells with phenotypic resistance that is not genotypically founded. These are the so-called persisters, which occur in in-vitro cultures at frequencies ranging from 1:106 to 1:108 (Fig. 3.30). The cause of such persistence is usually a specific metabolic property of these bacteria that prevents bactericidal substances from killing them. Following discontinuation of therapy, such persisters can lead to relapses. Infections with L-forms show a special type of persistence when treated with antibiotics that block murein synthesis .
Mechanisms of Action
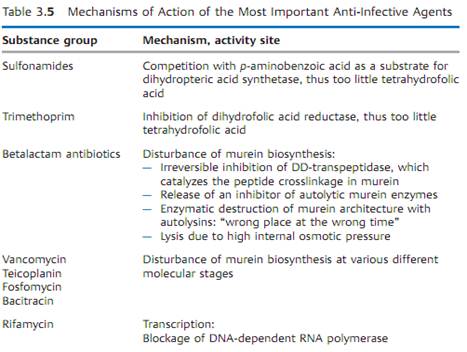
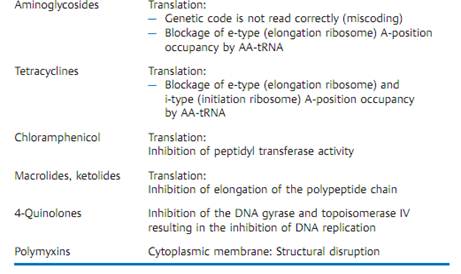
Table 3.5 provides a concise summary of the molecular mechanisms of action of the most important groups of anti-infective agents.
Pharmacokinetics
Pharmacokinetics covers the principles of absorption, distribution, and elimination of pharmacons by the macroorganism. The reader is referred to standard textbooks of pharmacology for details. The dosage and dosage interval recommendations for antibacterial therapy take into account the widely differing pharmacokinetic parameters of the different anti-infective agents, among them:
- Absorption rate and specific absorption time
- Volume of distribution
- Protein binding
- Serum (blood) concentration
- Tissue concentration
- Metabolization
- Elimination
Side Effects
Treatment with anti-infective agents can cause side effects, resulting either from noncompliance with important therapeutic principles or specific patient reactivity. On the whole, such side effects are of minor significance.
- Toxic effects. These effects arise from direct cell and tissue damage in the macroorganism. Blood concentrations of some substances must therefore be monitored during therapy if there is a risk of cumulation due to inefficient elimination (examples: aminoglycosides, vancomycin).
- Allergic reactions. See p. 108 for possible mechanisms (example: penicillin allergy).
- Biological side effects. Example: change in or elimination of normal flora, interfering with its function as a beneficial colonizer .
The Problem of Resistance
Definitions
Clinical resistance. Resistance of bacteria to the concentration of anti-infective agents maintained at the infection site in the macroorganism.
Natural resistance. Resistance characteristic of a bacterial species, genus, or family.
Acquired resistance. Strains of sensitive taxa can acquire resistance by way of changes in their genetic material.
Biochemical resistance. A biochemically detectable resistance observed in strains of sensitive taxa. The biochemical resistance often corresponds to the clinically relevant resistance. Biochemically resistant strains sometimes show low levels of resistance below the clinically defined boundary separat¬ing resistant and sensitive strains. Such strains may be medically susceptible.
Incidence, Significance
Problematic bacteria. Strains with acquired resistance are encountered fre-quently among Enterobacteriaceae, pseudomonads, staphylococci, and ente- rococci. Specific infection therapy directed at these pathogens is often fraught with difficulties, which explains the label problematic bacteria. They are responsible for most nosocomial infections . Usually harmless in otherwise healthy persons, they may cause life-threatening infections in highly susceptible, so-called problematic patients. Problematic bacteria are often characterized by multiple resistance. Resistance to anti-infective agents is observed less frequently in nonhospital bacteria.
Genetic variability. The basic cause of the high incidence of antibiotic resistance experienced with problematic bacteria is the pronounced genetic variability of these organisms, the mechanisms of which are described in the section “Genetic variability” . Most important are the mechanisms of horizontal transfer of resistance determinants responsible for the efficient distribution of resistance markers among these bacteria.
Selection. The origin and distribution of resistant strains is based to a significant extent on selection of resistance variants. The more often anti-infective substances are administered therapeutically, the greater the number of strains that will develop acquired resistance. Each hospital has a characteristic flora reflecting its prescription practice. A physician must be familiar with the resistance characteristics of this hospital flora so that the right anti-infective agents for a “calculated antibiotic therapy” can be selected even before the resistance test results are in. Such therapies take into account the frequency of infections by certain bacterial species (pathogen epidemiology) as well as current resistance levels among these bacteria (resistance epidemiology).
Resistance Mechanisms
Inactivating enzymes. Hydrolysis or modification of anti-infective agents.
- Betalactamases. Hydrolyze the betalactam ring of betalactam antibiotics (see Fig. 3.29). Over 200 different betalactamases are known. A course classification system is based on the substrate profile in penicillinases and cepha- losporinases. Production of some betalactamases is induced by betalactams , others are produced constitutively (unregulated).
- Aminoglycosidases. Modify aminoglycosides by means of phosphorylation and nucleotidylation of free hydroxyl groups (phosphotransferases and nucleotidyl transferases) or acetylation of free amino groups (acetyltrans-ferases).
- Chloramphenicol acetyltransferases. Modification, by acetylation, of chloramphenicol.
Resistant target molecules.
- Gene products with a low affinity to anti-infective agents are produced based on mutations in natural genes. Example: DNA gyrase subunit A, resistant to 4-quinolones.
- Acquisition of a gene that codes for a target molecule with low affinity to anti-infective agents. The resistance protein assumes the function of the sensitive target molecule. Example: methicillin resistance in staphylococci; acquisition of the penicillin-binding protein 2a, which is resistant to beta- lactam antibiotics and assumes the function of the naturally sensitive penicillin-binding proteins.
- Acquisition of the gene for an enzyme that alters the target structure of an anti-infective agent to render it resistant. Example: 23S rRNA methylases; modification of ribosomal RNA to prevent binding of macrolide antibiotics to the ribosome.
Permeability mechanisms.
- Reduced influx. Reduction of transport of anti-infective agents from outside to inside through membranes; rare.
- Increased efflux. Active transport of anti-infective agents from inside to outside by means of efflux pumps in the cytoplasmic membrane, making ef¬flux greater than influx; frequent.
Evolution of Resistance to Anti-Infective Agents
Resistance to anti-infective agents is genetically determined by resistance genes. Many resistance determinants are not new developments in response to the use of medical antibiotics, but developed millions of years ago in bacteria with no human associations. The evolutionary process is therefore a “nonanthropogenic” one. The determinants that code for resistance to anti-infective agents that are not antibiotics did develop after the substances began to be used in therapy, hence this is “anthropogenic” evolution. Factors contributing to the resistance problem have included the molecular mechanisms of genetic variability (mutation, homologous recombination, site-specific integration, transposition) and the mechanisms of intercellular gene transfer in bacteria (transformation, transduction, conjugation).
Resistance Tests
Two standard test systems are used to determine the in-vitro resistance levels of bacteria.
In dilution series tests, the minimum inhibitory concentration (MIC) of an anti-infective agent required to inhibit proliferation of a bacterial population is determined. A factor 2 geometrical dilution series of the agent is prepared in a nutrient medium, inoculated with the test organism and incubated, whereupon the lowest growth-inhibiting concentration level (mg/l) is determined. Three standardized dilution methods are available. In the agar dilution test, nutrient agar plates containing antibiotic are inoculated (“spotted”) with the test organisms. In the microbroth dilution test, the final volume is usually 100 il per microplate well. This test type can also be automated. The final volume in a macrobroth dilution test is 2 ml per tube.
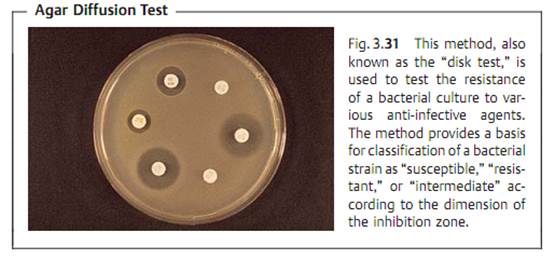
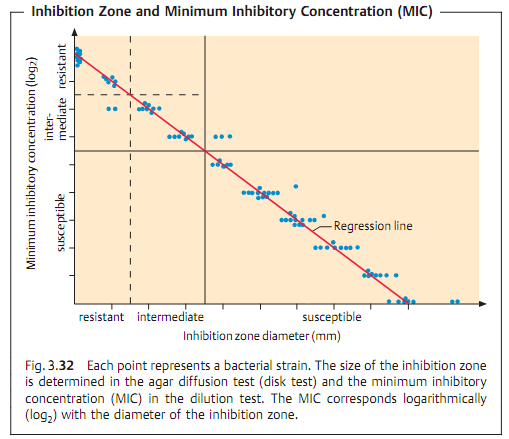
Due to the complexity and time-consuming nature of the above test types, routine laboratories often use the agar diffusion test. This involves diffuse inoculation of the nutrient agar plate with the test strain. Then disks of filter paper containing the anti-infective agents are placed on the agar. After the plates thus prepared are incubated, the inhibition zones around the disks (i.e., whether or not they develop and their size) provide information on the resistance of the microorganisms tested (Fig. 3.31). This is possible because of the linear relation between the log2 MIC and the diameter of the inhibition zones (Fig. 3.32).
To interpret the results, the MICs or inhibition zones are brought into relation with the substance concentrations present at a site of infection at standard dosage levels. This calculation is based on known averages for various pharmacokinetic parameters (serum concentration, half-life) and pharmacodynamics parameters (bactericidal activity or not, postantibiotic effect, etc.). The interpretation also takes into account clinical experience gained from therapy of infections with pathogens of given suceptibility. Such data are used to establish general guideline values defining the boundary between susceptible and resistant bacteria.
The minimum bactericidal concentration (MBC) is the smallest concentration of a substance required to kill 99.9% of the cells in an inoculum.
The MBC is determined using quantitative subcultures from the macro-scopically unclouded tubes or (microplate) wells of an MIC dilution series.
Combination Therapy
Combination therapy is the term for concurrent administration of two or more anti-infective agents. Some galenic preparations combine two components in a fixed ratio (example: cotrimoxazole). Normally, however, the in dividual substances in a combination therapy are administered separately. Several different objectives can be pursued with combination therapy:
- Broadening of the spectrum of action. In mixed infections with pathogens of varying resistance; in calculated therapy of infections with unknown, or not yet known, pathogenic flora and resistance characteristics.
- Delay of resistance development. In therapy of tuberculosis; when using anti-infective agents against which bacteria quickly develop resistance.
- Potentiation of efficacy. In severe infections requiring bactericidal activity at the site of infection. Best-known example: penicillin plus gentamicin in treatment of endocarditis caused by enterococci or streptococci.
Combining the effects of anti-infective drugs can have several different effects:
- No difference. The combination is no more efficacious than the more active of the two components alone.
- Addition. Summation of the effects.
- Synergism. Potentiation of the effects.
- Antagonism. The combination is less efficacious than one of the two components alone.
Rule of thumb: combinations of bacteriostatics with substances that are bactericidal in the cell division phase only often result in antagonism, e.g., penicillin plus tetracycline in therapy of pneumococcal pneumonia.
In-vitro investigations of the mechanism of action of a combination when used against a pathogen usually employ the so-called “checkerboard titration” technique, in which the combinatory effects of substances A and B are compared using a checkerboard-like pattern.
Chemoprophylaxis
One of the most controversial antibiotic uses is prophylactic antibiosis. There are no clear-cut solutions here. There are certain situations in which chemoprophylaxis is clearly indicated and others in which it is clearly contraindicated. The matter must be decided on a case-by-case basis by weighing potential benefits against potential harm (side effects, superinfections with highly virulent and resistant pathogens, selection of resistant bacteria).
Chemoprophylaxis is considered useful in malaria, rheumatic fever, pulmonary cystic fibrosis, recurring pyelonephritis, following intensive contact
with meningococci carriers, before surgery involving massive bacterial contamination, in heavily immunocompromised patients, in cardiac surgery or in femoral amputations due to circulatory problems. Chemoprophylaxis aimed at preventing a postsurgical infection should begin a few hours before the operation and never be continued for longer than 24-72 hours.
Immunomodulators
Despite the generally good efficacy of anti-infective agents, therapeutic success cannot be guaranteed. Complete elimination of bacterial pathogens also requires a functioning immune defense system. In view of the fact that the number of patients with severe immunodeficiencies is on the rise, immunomodulators are used as a supportive adjunct to specific antibiotic therapy in such patients. Many of these “cytokines” produced by the cells of the immune system can now be produced as “recombinant proteins.” Mye-lopoietic growth factors have now been successfully used in patients suffering from neutropenia. Additional immunomodulators are also available, e.g., interferon gamma (IFNy) and interleukin 2 (IL-2).



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|