


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-9-2017
Date: 20-9-2017
Date: 12-1-2020
|
THERMAL BEHAVIOR
For engineering purposes, the most useful classification of polymers is based on their thermal (thermomechanical) response. Under this scheme, polymers are classified as thermoplastics or thermosets. As the name suggests, thermoplastic polymers soften and flow under the action of heat and pressure. Upon cooling, the polymer hardens and assumes the shape of the mold (container). Thermoplastics, when compounded with appropriate ingredients, can usually withstand several of these heating and cooling cycles without suffering any structural breakdown. This behavior is similar to that of candle wax. Examples of thermoplastic polymers are polyethylene, polystyrene, and nylon.
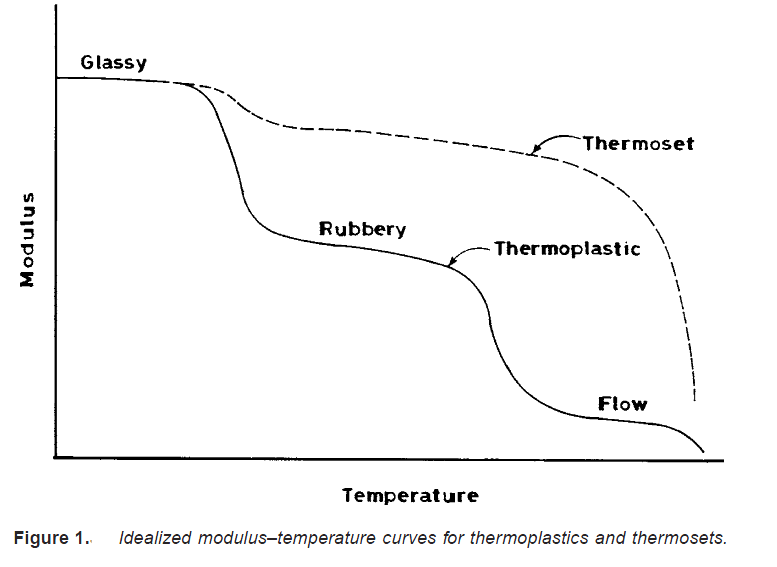
A thermoset is a polymer that, when heated, undergoes a chemical change to produce a cross-linked, solid polymer. Thermosets usually exist initially as liquids called prepolymers; they can be shaped into desired forms by the application of heat and pressure, but are incapable of undergoing repeated cycles of softening and hardening. Examples of thermosetting polymers include urea–formaldehyde, phenol–formaldehyde, and epoxies.
The basic structural difference between thermoplastics and thermosets is that thermoplastic polymers are composed mainly of linear and branched molecules, whereas thermosets are made up of cross-linked systems. Recall from our previous discussion that linear and branched polymers consist of molecules that are not chemically tied together. It is therefore possible for individual chains to slide past one another. For cross-linked systems, however, chains are linked chemically; consequently, chains will not flow freely even under the application of heat and pressure.
The differences in the thermal behavior of thermoplastics and thermosets are best illustrated by considering the change in modulus with temperature for both polymers (Figure 1). At low temperatures, a thermoplastic polymer (both crystalline and amorphous) exists as a hard and rigid glass. As the temperature is increased, it changes from a glass to a rubbery elastomer to a viscous melt that is capable of flowing — hence this phase is also known as the flow region. (The transitions between the different phases or regions of thermal behavior are characterized by drops in the magnitude of the modulus — usually two to three orders. As we shall see later, differences exist between amorphous and crystalline thermoplastics in the details and nature of these transitions). For the thermosetting polymer, on the other hand, the modulus remains high in the rubbery region, while the flow region disappears.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|