
 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-12-2015
Date: 16-5-2016
Date: 21-11-2015
|
Angiogenin
Angiogenin (Ang) is one of a group of proteins that are potent inducers of angiogenesis, the process by which new blood vessels are formed. It was first isolated from medium conditioned by human adenocarcinoma (HT-29) cells based on the premise that tumor cells must secrete angiogenic factors in order to attract blood vessels so that they can grow. Ang was identified by its ability to stimulate angiogenesis on the chorioallantois, the outer membrane that surrounds the embryo in fertilized chicken eggs. Subsequently, it was shown to be present in normal human plasma, bovine milk, and bovine and mouse serum. It is a 14-kDa basic protein that has 33% sequence identity to bovine pancreatic ribonuclease A and is one of several members of the RNase A superfamily of proteins that have unusual biological activities. It is the only angiogenic protein that is a ribonuclease, and the only ribonuclease that is angiogenic. It is an important protein that may be involved in wound healing, is critical for the growth of solid tumors, and has become a target for the treatment of metastatic cancer and other angiogenesis-related diseases. For recent reviews on Ang see Refs. 1 and 2.
1. Protein Chemistry
Human Ang is a single polypeptide chain of 123 amino-acid residues and three disulfide bonds (Fig .( 1. All of the main components of the catalytic site (see Active Site) of RNase A are present in Ang, and thus it is not surprising that it has enzymatic activity toward RNA and dinucleotides and which, like that of RNase A, is limited to cleavage after pyrimidines. What is surprising is that this activity is four to six orders of magnitude less than that of RNase A. Nevertheless, it is essential for angiogenic activity. Part of the reason for such low activity was revealed by X-ray crystallography (Fig. 2). The structure of Ang is closely similar to that of RNase A, but with two major differences. On the one hand, the pyrimidine-binding (B1) component of the catalytic active site was found to be occluded by the side chain of Gln117. On the other, the second (B2) component of this site is structurally quite different from that in RNase A. In the latter, it is part of an eight-residue disulfide loop that is found in all of the 55 known mammalian members of the ribonuclease superfamily but missing in all of the nine known Ang. Instead, it is replaced by a sequence of residues that interacts with a cell-surface binding protein. Despite these likely impediments to substrate binding, Ang does catalyze RNA hydrolysis, albeit very weakly.
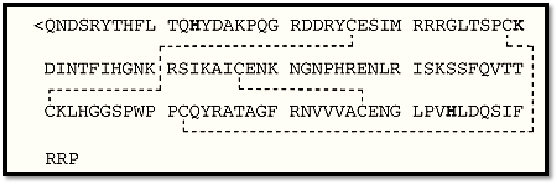
Figure 1. Amino acid sequence of human angiogenin. The active site histidine (H) and lysine (K) residues are in bold, and the disulfide bridges between cysteine (C) residues are indicated by dotted lines.

Figure 2. The polypeptide fold for human angiogenin drawn with the program MOLSCRIPT. (From Ref. 9, with permission).
It has been suggested that through evolution Ang was endowed with a unique structure that attenuates its catalytic potency toward RNA in general, but that undergoes a conformational change when Ang is brought together with its specific, yet unidentified, substrate (3). Whether this structural rearrangement activates Ang or merely ensures its specificity is unknown. It may be that the very low activity of Ang is perfectly adequate for carrying out its unique biological function. It should be noted, however, that Ang is an effective inhibitor of cell-free protein synthesis by virtue of its ability to cleave 18S rRNA when this substrate is present in the intact ribosome, and in fact is an even more effective inhibitor than RNase A. When isolated 18S RNA is used as substrate, the activity of Ang is markedly decreased relative to RNase A. This suggests that the activity of Ang depends on the environment of its substrate.
2. Molecular Biology
The human Ang gene, localized to chromosome band 14q11, is present as a single copy per haploid genome, with no introns in the protein-coding region (1, 2). The gene has been cloned and expressed in both Escherichia coli and BHK cells, and well over 40 Ang variants have been prepared in order to explore various aspects of its structure–function relationships. Thus, site-directed mutagenesis of His13, His114, or Lys40 (the equivalents of the catalytic residues His12, His119, and Lys41 in RNase A) abolishes both the ribonucleolytic and the angiogenic activity of Ang. Although angiogenically inactive, these variants block the angiogenic activity of native angiogenin. By contrast, an Ang variant in which residues 58 to 70 were replaced by residues 59 to 73 of RNase A had 300- to 600-fold more activity toward RNA substrates but was angiogenically inactive. Thus, ribonuclease activity is essential but not sufficient for angiogenic activity. Moreover, this variant, unlike the active site variants, was unable to block the angiogenic activity of native Ang, suggesting that the mutated region of the protein is involved in cell binding, probably interacting with a cell-surface binding protein. Recall that in RNase A this region of the molecule constitutes the B2 component of the substrate binding site. In Ang it apparently has evolved into a cell binding site, with loss of catalytic activity but concomitant acquisition of a new biological potential.
3. Cell Biology
Blood vessels are composed of specialized cells known as endothelial cells. It is not surprising, given its function, that 125I-labeled Ang binds to endothelial cells, and it does so in a manner that is characteristic of receptor binding—that is, time- and concentration-dependent, reversible, saturable, and competitive with unlabeled Ang. Yet Ang is a plasma protein (200 to 400 mg/L), and obviously it does not continuously stimulate new blood vessel formation as it circulates through the vasculature. This is explained by the fact that the angiogenin receptor is only expressed in sparsely cultured endothelial cells, not in confluent cells (as exist in blood vessels) (4). The receptor, not yet fully characterized, is a 170-kDa transmembrane protein whose presence on the cell surface correlates with the mitogenic activity of Ang. When the receptor is present, the cells respond to Ang by both increased thymidine uptake and cell proliferation. Confluent cells lack the receptor and, hence, do not respond this way to Ang.
Experiments with protein cross-linking reagents have demonstrated that Ang also interacts with a 42-kDa endothelial cell-surface protein that is a member of the actin family (1, 2). On binding Ang, it dissociates from the cell surface as an Ang–actin complex. Remarkably, this complex activates tissue plasminogen activator (tPA) and thus generates plasmin (see Plasminogen). This, in turn, stimulates cell-associated proteolytic activity to degrade the extracellular matrix and thereby facilitates cell migration, an essential feature of the angiogenesis process. Ang also acts as a cell adhesion molecule and, when coated on a plastic surface, mediates the binding of endothelial and tumor cells. In vivo it may help direct migrating blood vessels toward Ang-secreting tumor cells.
Addition of Ang to cultured endothelial cells stimulates a transient increase in cellular diacylglycerol, seemingly the result of an Ang-induced activation of phospholipase C, as well as an increase in prostacyclin secretion owing to activation of phospholipase A2. Interpretation of these second messenger responses has not been possible, thus far, largely because the systems are complex and may only be stimulated indirectly by Ang.
4. Nuclear Translocation
Angiogenin undergoes endocytosis by sparsely cultured endothelial cells and is rapidly translocated from the cell surface to the nucleus, where it accumulates in the nucleolus (1, 2). This process is receptor-mediated and regulated by a nuclear localization signal that involves three basic residues, Arg31-Arg32-Arg33, of Ang. This signal is essential for angiogenesis and suggests that the substrate for the ribonucleolytic activity of Ang is located in the nucleolus, a highly specialized region where biogenesis of ribosomes takes place (see Nucleolus and Ribosomes). One possibility is that the ribonucleolytic activity of Ang enhances the transcription and processing of rRNA. Two enzymatically inactive variants of angiogenin whose cell-binding site is intact also accumulate in the nucleolus when added to endothelial cells, but they are angiogenically inactive. This suggests that not only is nuclear localization essential for the biological activity of Ang but, in addition, the translocated protein must be enzymatically active. This has been confirmed by recent studies in which a DNA aptamer (an oligonucleotide that binds fairly tightly to Ang) that inhibits both the enzymatic and angiogenic activities of Ang is also translocated to nucleus, but only when added to endothelial cells together with Ang. The inhibitor and the protein accumulate in the nucleus in a 1:1 stoichiometric ratio (5).
5. Mechanism of Action
Although Ang may well be involved in a variety of angiogenesis-related situations, its mechanism of action is likely to be quite similar for each of them; hence it is only summarized here in the context of tumor-induced angiogenesis. All tumor cells that have been examined have been found to secrete Ang, and in at least one case, pancreatic cancer, the aggressiveness of the tumor is related to the amount of angiogenin produced (6). Ang secreted by tumor cells migrates through the extracellular matrix until it reaches an endothelial cell. There it combines with cell-surface actin and dissociates as a complex that stimulates the activity of tPA and the formation of plasmin. Degradation of the extracellular matrix—for example, by plasmin-activated matrix metalloproteinases—may stimulate a few endothelial cells to migrate, and this could trigger expression of the Ang receptor. Binding of Ang to the receptor would then, on the one hand, initiate a second-messenger response, perhaps through activation of the above-mentioned phospholipases, and, on the other, promote endocytosis and nuclear localization of Ang. The ribonucleolytic action of Ang within the nucleolus would, together with signals generated via the second messengers, activate processes leading to cell proliferation. The proliferating endothelial cells would migrate through the now degraded extracellular matrix toward the tumor cell from which the Ang was released. The cell-adhesion properties of Ang may be important for ensuring cell migration in the proper direction. At present, this view of the mode of action of Ang is largely speculative, particularly because nothing is known about the events that occur within the nucleus and lead to cell division. Nevertheless, it summarizes current thinking and is a useful basis for further investigation.
6. Tumor Biology
The levels of Ang and its messenger RNA are increased in the tissues and cells of patients with various types of cancers, indicative of an in vivo role for Ang in the process of tumor angiogenesis. This being the case, anti-angiogenin agents could have potential importance in the treatment of cancer and other angiogenesis-related diseases. The most potent inhibitor of Ang is a 50-kDa protein originally isolated from placenta as an inhibitor of RNase A, hence known as placental ribonuclease inhibitor (RI) (1, 2). It inhibits Ang with a Ki of 0.7 fM, one of the strongest protein–protein interactions known, and is 60-fold stronger than its inhibition of RNase A. Unfortunately, RI is evidently unstable in extracellular fluids and has not been found to be effective in treating tumors in laboratory animals.
Monoclonal antibodies raised against Ang have been used to treat athymic mice injected with HT-29 colon carcinoma cells, and they reduced the incidence of tumors by up to 65%. Similar results have been observed with other types of tumors, and when used in conjunction with conventional therapeutic agents, a synergistic effect was seen (2). Actin was also tested for its anti-tumor activity, and it too was capable of preventing tumor growth in more than 60% of treated animals. It should be noted that anti-angiogenin agents do not affect the growth of already-established tumors. They are only effective when administered to animals at the same time as the tumor cells. They are thought to act by specific extracellular inactivation of tumor-secreted Ang and the consequent inhibition of tumor angiogenesis. These experiments are important in that they provide clear evidence of a crucial role for angiogenin in the early stages of development of these tumors.
7. Other Biological Properties
One of the major complications associated with regular hemodialysis is the increased morbidity and mortality arising from infections. This has been attributed to dysfunction of polymorphonuclear leukocytes. Indeed, a number of compounds have been isolated from uremic serum and shown to inhibit the biological activity of these white cells. One of these is an inhibitor of leukocyte degranulation that turns out to be Ang (7). Nanomolar concentrations of Ang inhibit both spontaneous and peptide-stimulated degranulation by 60% and 30%, respectively. However, Ang has no other effect on the cellular responses of polymorphonuclear leukocytes, such as chemotaxis, phagocytosis or their peptide-stimulated oxidative respiratory burst.
Ang has also been reported to suppress significantly the proliferation of human lymphocytes stimulated by a mixed-lymphocyte culture or by phytohemagglutinin or concanavalin A (8). A maximal immunosuppressive effect was seen at an Ang dose of 50 to 100 mg/mL. It was thought that this effect might synergize with the effect of Ang on neovascularization of tumors and thereby contribute to tumor development.
8. Conclusions
Ang is now recognized as a pleiotropic molecule capable of inducing several intra- and extracellular activities. It induces most of the individual events in the process of angiogenesis including binding to endothelial cells, stimulating second messengers, mediating cell adhesion, activating cell-associated proteinases, inducing cell invasion, stimulating DNA synthesis and cell proliferation, and organizing the formation of tubular structures from cultured endothelial cells. Characterization of its cellular receptors, elucidation of its mechanism of nuclear translocation, identification of its intranucleolar target substrate, and understanding how these events result in cellular proliferation and blood vessel formation will provide important and novel information that can be utilized for either promoting or inhibiting angiogenesis for therapeutic purposes.
References
1. J. F. Riordan (1997) "Structure and Function of Angiogenin" In Ribonucleases: Structures and Functions (G. D''Alessio and J. F. Riordan, eds.), Academic Press, New York, pp. 445–489.
2. B. L. Vallee and J. F. Riordan (1997) Cell. Mol. Life Sci. 53, 803–815.
3. R. Shapiro (1998) Biochemistry 37, 6847–6856.
4. G.-f. Hu, J. F. Riordan, and B. L. Vallee (1997) Proc. Natl. Acad. Sci. USA 94, 2204–2209.
5. V. Nobile, N. Russo, G.-f. Hu, and J. F. Riordan (1998) Biochemistry 37, 6857–6863.
6. S. Shimoyama et al. (1996) Cancer Res. 56, 2703–2706.
7. H. Tschesche, C. Kopp, W. H. Horl, and U. Hempelmann (1994) J. Biol. Chem. 269, 3027430280- .
8. J. Matousek et al. (1995) Comp. Biochem. Physiol. 112B, 235–241.
9. P. J. Kraulis (1991) J. Appl. Crystallogr. A 24, 946–950.



|
|
|
|
حمية العقل.. نظام صحي لإطالة شباب دماغك
|
|
|
|
|
|
|
إيرباص تكشف عن نموذج تجريبي من نصف طائرة ونصف هليكوبتر
|
|
|
|
|
|
ستوفر فحوصات تشخيصية لم تكن متوفرة سابقا... تعرف على مميزات أجهزة المختبر في مستشفى الثقلين لعلاج الاورام في البصرة
|
|
|
|
بالصور: تزامنا مع ذكرى ولادة الإمام الرضا (ع).. لوحات مطرزة تزين الصحن الحسيني الشريف
|
|
|
|
بالفيديو: الاكبر في العراق.. العتبة الحسينية تنجز المرحلة الأولى من مدينة الثقلين لإسكان الفقراء في البصرة
|
|
|
|
ضمنها مقام التل الزينبي والمضيف.. العتبة الحسينية تعلن عن افتتاحها ثلاثة أجزاء من مشروع صحن العقيلة زينب (ع) خلال الفترة المقبلة
|