


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Enhancement of Leaving Group Abilities by Electrophilic Catalysis |
|
|
|
أقرأ أيضاً
التاريخ: 23-12-2021
التاريخ: 3-4-2017
التاريخ: 2024-04-21
التاريخ: 25-5-2017
|
In general, a leaving group that leaves as a neutral molecule is a much better leaving group than one that leaves as an anion. Alcohols, ROH, are particularly unreactive in SN reactions because OH⊖ is a very poor leaving group. However, if a strong acid is present, the reactivity of the alcohol is enhanced greatly. The acid functions by donating a proton to the oxygen of the alcohol, thereby transforming the hydroxyl function into ROH2⊕, which has a much better leaving group, H2O, in place of OH⊖. The SN reactions of ethers and esters are acid-catalyzed for the same reason.
Heavy-metal salts, particularly those of silver, mercury, and copper, catalyze SN1 reactions of alkyl halides in much the same way that acids catalyze the SN reactions of alcohols. A heavy-metal ion functions by complexing with the unshared electrons of the halide, thereby making the leaving group a metal halide rather than a halide ion. This acceleration of the rates of halide reactions is the basis for a qualitative test for alkyl halides with silver nitrate in ethanol solution:
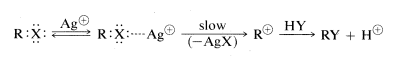
Silver halide precipitates at a rate that depends upon the structure of the alkyl group, tertiary > secondary > primary. Tertiary halides usually react immediately at room temperature, whereas primary halides require heating. That complexes actually are formed between organic halides and silver ion is indicated by an increase in water solubility in the presence of silver ion for those halides that are slow in forming carboncations.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|