


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 18-12-2021
التاريخ: 16-12-2021
التاريخ: 16-12-2021
التاريخ: 16-12-2021
|
Some Philosophical Observations
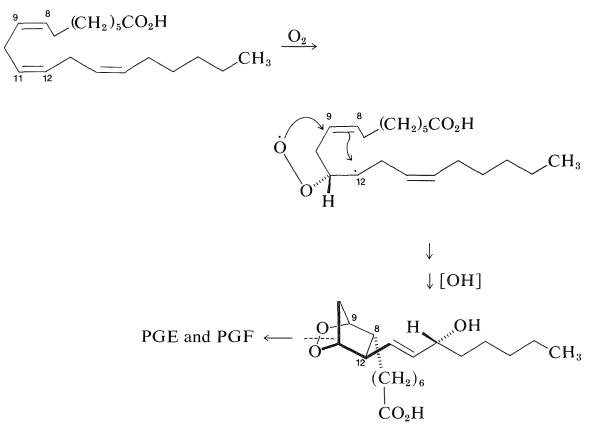
Prostaglandins are found in low concentrations distributed in a large number of organs, tissues, and body fluids of mammals. They exhibit a broad spectrum of physiological activity and are remarkably potent. Their precise biological role is not entirely clear, but they are known to induce strong contractions of smooth muscle tissue (lungs, uterus) and to lower blood pressure and sodium levels. Prostaglandins also have been implicated in the control of pituitary hormones released from the hypothalamus, and in the incidence of "pain" as a response to fever and inflammation. In fact, the analgesic property of aspirin possibly may result from the inhibition of prostaglandin biosynthesis. Although prostaglandins are not yet in extensive clinical use, their wide-ranging physiological effects hold promise that they will become useful drugs for the treatment of high blood pressure, thrombosis, respiratory disease, hypertension, ulcers, and in the regulation of fertility in both men and women.
A number of brilliant total syntheses of natural prostaglandins have been developed and these also have provided a number of interesting prostaglandin analogs. The biosynthesis of prostaglandins proceeds by oxygenation at C11 of unsaturated fatty acids. This is followed by cyclization (probably as the result of a radical addition mechanism) to a bicyclic peroxide. Cleavage of the peroxide ring leads to prostaglandins:
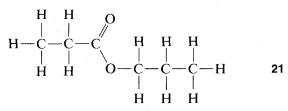
As you proceed with your study of organic chemistry, you may well feel confused as to what it is you are actually dealing with. On the one hand, there will be exhortations to remember how organic chemistry pervades our everyday life. And yet, on the other hand, you also will be exhorted to think about organic compounds in terms of abstract structural formulas representing molecules when there is absolutely no way at all to deal with molecules as single entities. Especially if you are not studying organic compounds in the laboratory concurrently, you may come to confuse the abstraction of formulas and ball-and-stick models of the molecules with the reality of organic compounds, and this would be most undesirable. At each stage of the way, you should try to make, or at least visualize, a juncture between a structural formula and an actual substance in a bottle. This will not be easy - it takes time to reach the level of experience that a practicing organic chemist has so that he can tell you with some certainty that the structural formula 21 represents in actuality, a limpid, colorless liquid with a pleasant odor, slightly soluble in water, boiling somewhere about 100o100o.
A useful method for developing this sort of feeling for the relationship between structures and actual compounds is to check your perception of particular substances with their properties as given in a chemical handbook.
One, perhaps comforting, thought for you at this time is that differences between the chemical behaviors of relatively similar organic compounds usually are ascribed to just three important and different kinds of effects - two of which have root in common experience. One, called steric hindrance, is a manifestation of experience that two solid objects cannot occupy the same space at once. Another is the electrical effect, which boils down to a familiar catechism that like electrical charges repel each other and unlike charges attract each other. The remaining important effect, the one that has no basis in common experience, derives from quantum mechanics. The quantum mechanical effect explains why benzene is unusually stable, how and why many reactions occur in special ways and, probably most important of all, the ways that organic compounds interact with electromagnetic radiation of all kinds - from radio waves to x rays.
We shall try to give as clear explanations as possible of the quantum mechanical effects, but some of it will just have to be accepted as fact that we cannot ourselves experience directly nor understand intuitively. For example, when a grindstone rotates, so far as our experience goes, it can have an infinitely variable rate of rotation and, consequently, infinitely variable rotation (angular) momentum. However, molecules in the gas phase have only specific rotation rates and corresponding specific rotational momentum values. No measurement technique can detect in-between values of these quantities. Molecules are "quantized rotators." About all you can do is try to accept this fact, and if you try long enough, you may be able to substitute familiarity for understanding and be happy with that.
All of us have some concepts we use continually (even perhaps unconsciously) about energy and work. Thermodynamics makes these concepts quantitative and provides very useful information about what might be called the potential for any process to occur, be it production of electricity from a battery, water running uphill, photosynthesis, or formation of nitrogen oxides in combustion of gasoline. In the past, most organic chemists seldom tried to apply thermodynamics to the reactions in which they were interested. Much of this was due to the paucity of thermodynamic data for more than a few organic compounds, but some was because organic chemists often liked to think of themselves as artistic types with little use for quantitative data on their reactions (which may have meant that they didn't really know about thermodynamics and were afraid to ask).
Times have changed. Extensive thermochemical data are now available, the procedures are well understood, and the results both useful and interesting. We shall make considerable use of thermodynamics in our exposition of organic chemistry. We believe it will greatly improve your understanding of why some reactions go and others do not.
Finally, you should recognize that you almost surely will have some problems with the following chapters in making decisions as to how much time and emphasis you should put on the various concepts, principles, facts, and so on, that we will present for you. As best we can, we try to help you by pointing out that this idea, fact, and so on, is "especially important," or words to that effect. Also, we have tried to underscore important information by indicating the breadth of its application to other scientific disciplines as well as to technology. In addition, we have caused considerable material to be set in smaller type and indented. Such material includes extensions of basic ideas and departments of fuller explanation. In many places, the exposition is more complete than it needs to be for you at the particular location in the book. However, you will have need for the extra material later and it will be easier to locate and easier to refresh your memory on what came before, if it is one place. We will try to indicate clearly what you should learn immediately and what you will want to come back for later.
The problem is, no matter what we think is important, you or your professor will have your own judgments about relevance. And because it is quite impossible to write an individual text for your particular interests and needs, we have tried to accommodate a range of interests and needs through providing a rather rich buffet of knowledge about modern organic chemistry. Hopefully, all you will need is here, but there is surely much more, too. So, to avoid intellectual indigestion, we suggest you not try to learn everything as it comes, but rather try hardest to understand the basic ideas and concepts to which we give the greatest emphasis. As you proceed further, the really important facts, nomenclature, and so on (the kind of material that basically requires memorization), will emerge as that which, in your own course of study, you will find you use over and over again. In hope that you may wish either to learn more about particular topics or perhaps gain better understanding through exposure to a different perspective on how they can be presented, we have provided supplementary reading lists at the end of each chapter.
Our text contains many exercises. You will encounter some in the middle of the chapters arranged to be closely allied to the subject at hand. Others will be in the form of supplementary exercises at the end of the chapters. Many of the exercises will be drill; many others will extend and enlarge upon the text. The more difficult problems are marked with a star (∗).



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|