


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-12-2015
Date: 3-6-2021
Date: 9-6-2021
|
Gene Targeting by Homologous Recombination in Embryonic Stem Cells
Gene targeting was developed to ablate (knock out) the function of a specific gene. Although gene targeting is still most commonly used to generate this type of mutation, the method is now the basis of many techniques which can produce a whole variety of DNA modifications to
Table .1 Advantages and disadvantages of generating transgenic mice by pronuclear injection of DNA.

the mouse genome, including the deletion/rearrangement of large regions of chromosomes containing multiple genes, the introduction of subtle mutations into the genome the knock-in of a gene of interest and conditional gene targeting .
The technique of gene targeting in the mouse has enabled major advances to be made in our understanding of mammalian gene function, in normal developmental, biochemical and physiological processes, and also in disease processes. The technique was established by bringing together two important findings (i) that homologous recombination can be used to modify specifically a target mammalian gene and (ii) that embryonic stem cells isolated from pre-implantation stage mouse embryos can be maintained in culture and then used to contribute to the germline of another mouse. The importance of this technique to biomedical research was acknowledged when Mario R. Capecchi, Martin J. Evans and Oliver Smithies jointly received the Nobel Prize in Physiology or Medicine 2007 for their discoveries of the ‘principles for introducing specific gene modifications in mice by the use of embryonic stem cells’.
1. Basic Principles
1.1 Homologous Recombination
Homologous recombination is used to introduce modifications into a gene by exchanging endogenous DNA sequences in the chromosome with cloned DNA sequences (targeting vector). Gene targeting by homologous recombination in mammalian cells was first reported in 1985 allowing for the stable insertion of DNA into the genome at a predictable site.
1.2 Embryonic Stem Cells
Embryonic stem (ES) cells are isolated from the inner cell mass of preimplantation stage embryos at 3.5 days post coitum; at this stage embryos are known as blastocysts. The crucially important factor about the progenitor cells of these early embryos is that they are pluripotent –
they have the potential to differentiate into any cell type, including the germ cells, of the subsequent embryo. It was in 1986 that Evans and colleagues demonstrated that these ES cells could be maintained in culture, where they could be genetically modified and then used to repopulate the germline of another mouse, thereby generating chimeric offspring containing genetic material from both the recipient mouse and the ES cells. The chimeric mice, when mated, could pass on the genetic mutation to the next generation; with the mutation being inherited according to Mendel’s laws of genetics.
It is interesting to note that the ability of ES cells to populate the germline has so far only been identified in the mouse and not even in all mouse strains.
Smithies, Capecchi and their colleagues brought together the homologous recombination and ES cell technologies to develop the process known as gene targeting. It is essential that ES cells to be used in gene targeting experiments maintain their pluripotency in order to contribute to the germline of chimeric mice. It has been found that the presence of the cytokine leukaemia inhibitory factor (LIF) is essential to ensure that ES cells do not differentiate in vitro. For this reason, ES cells are generally grown on a feeder layer of fibroblasts which secrete LIF into the culture medium. When ES cells are grown in optimal culture conditions, they grow as discrete colonies of densely packed cells, with no obvious sign of differentiation (Figure 1). Other culture conditions have been shown to be essential in maintaining the ES cells in their undifferentiated state, including growing at a high cell density and ensuring that the colonies are thoroughly disaggregated when passaging (splitting) the cells, since clumps of cells are likely to differentiate.
Most ES cells lines currently in use have been derived from the 129 strain of mouse which has an agouti coat colour genotype ; this is useful when identifying chimeric mice (see step 5 below). Another interesting feature of many of the ES cell lines currently in use is that they have an XY (male) genotype, which leads to the production of mainly male chimeras (even when XY ES cells are injected into a female blastocyst the resulting chimeric pup will tend to be male).
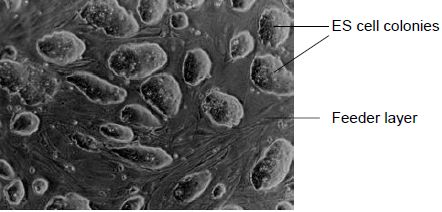
Figure 1. ES cell colonies growing on a layer of fibroblast feeder cells. Healthy, undifferentiated ES cells form densely packed colonies.
2. Generation of a Knockout Mouse
As with transgenesis by pronuclear injection, gene targeting by homologous recombination in embryonic stem cells is a multi-step process. It begins with the generation of the targeting vector, which is transferred by electroporation into the ES cells. The ES cells are cultured and analysed for the presence of the homologously recombined DNA sequence; the targeted ES cells are then injected into blastocyst stage embryos. The resultant pups are screened for the level of chimerism (percentage contribution from the targeted ES cells) and the chimeric mice are then tested for their ability to pass the targeted mutation through the germline and to generate mice heterozygous for the mutation. Heterozygous mice can then be mated to generate the homozygous knockout mouse (Figure 2).
Step 1. Generation of a Targeting Vector
When designing and constructing a targeting vector, a number of factors must be considered which will influence the type of mutation to be
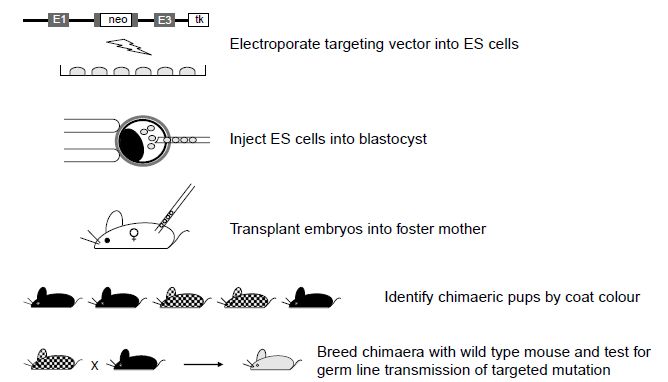
Figure2 Generation of gene knockout mice by gene targeting in ES cells. The targeting vector is electroporated into the ES cells. ES cells that have undergone homologous recombination are injected into blastocyst stage embryos and these embryos are then transplanted to pseudo-pregnant foster mothers. Chimeric offspring can be identified by their coat colour; these pups will carry the targeted mutation carried by the injected ES cells. Chimeric offspring can then be mated to wild type mice to determine whether they transmit the targeted mutation through the germline to give pups heterozygous for the mutation. The heterozygous offspring can then be intercrossed to mice homozygous for the mutation.
introduced, the efficiency of targeting and the ease with which successful targeting can be detected. DNA homologous with the chromosomal/gene site of interest. For successful and efficient targeting, the vector must contain at least 5–10 kb of isogenic DNA homologous with the sequence to be targeted. This homologous sequence is divided between the short arm of homology (1–1.5 kb) and a long arm of homology (4–8 kb); this permits easy screening of the ES clones. It is ideal to identify gene targeted colonies by PCR designed to span the short arm of homology. It is known that the efficiency of homologous recombination is decreased when there are base pair differences between the donor and recipient DNA. For this reason, it is now common practice for the DNA used to construct the targeting vector to originate from the same mouse strain as the ES cells (i.e. isogenic DNA).
Positive and negative selection cassettes. Since gene targeting by homologous recombination occurs at low frequencies (typically 10-5–10-6 of ES cells treated with construct DNA) and the targeting construct is much more likely to insert randomly into the genome, it is essential to be able to screen ES cell colonies quickly and efficiently for successful targeting.
For this reason, most targeting vectors will be designed to insert a positive selection cassette into the gene of interest. For example, the neomycin phosphotransferase gene (neo) is often used as a positive marker, which when expressed in the ES cell genome will render the cells resistant to treatment with the antibiotic neomycin sulfate (G418). A negative selection marker can also be used to enrich for gene targeted colonies. The selection marker is cloned outside of the homologous sequence in the targeting vector and will therefore not insert into the genome when homologous recombination occurs, but will insert into the genome if random integration of the targeting vector occurs. For example, the herpes simplex virus thymidine kinase gene (HSVtk) when expressed in ES cells will produce a toxic product in the presence of gancyclovir (a thymidine analogue), killing ES cells expressing this gene (Figure 3).
Step 2. ES Cell Transfection
The most efficient method for introducing the targeting vector into the ES cells is by electroporation. The linearised vector DNA is electroporated into a large number of ES cells in a single cell suspension; the cells are then plated on to fresh feeder cells. Then, 24 h after electroporation, the selection process can begin, which will kill cells which have not incorporated the targeting vector by homologous recombination. The ES cells are cultured in media containing the drugs used for selection for 7–10 days; this will enrich the population with cells that have undergone homologous recombination; however, it must be noted that this process is not 100% efficient.
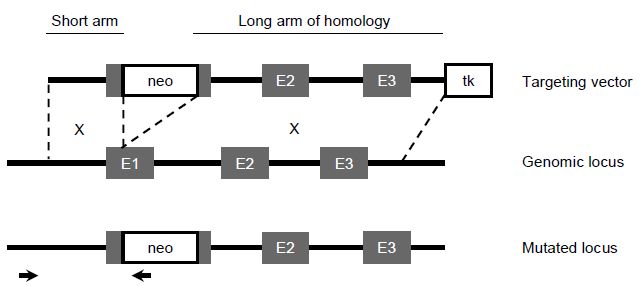
Figure 3 Gene targeting strategy. The top panel depicts a conventional targeting vector in which the positive selection cassette (neo) has been cloned into the first exon (E1) of the target gene and the negative selection cassette has been cloned at the end of the targeting construct after exons 2 and 3 (E2 and E3). The middle panel represents the genomic locus and homologous recombination will take place between the genomic locus and the two arms of homologous sequence in the targeting vector. The crossover points are depicted by the X. The bottom panel depicts the mutated locus when homologous recombination has occurred. The two small arrows represent the PCR primers which will be used to amplify the sequence across the short arm of homology to identify ES cells which have undergone homologous recombination.
Step 3. Identification of ES Cells Targeted by Homologous Recombination
To identify the ES cells that have undergone gene targeting by homologous recombination, discrete colonies are identified and picked. The colonies are dissociated into single cells by treatment with trypsin, divided between two wells on duplicate microtitre plates and cultured. The purpose of dividing the cells between duplicate plates is to allow one plate of cells to be used to prepare DNA to identify targeted ES cells and the cells from the second plate can be used to inject into blastocysts. Genomic DNA is prepared from each ES cell clone, which is then screened by PCR to identify clones in which homologous recombination has occurred. It is likely that several hundred ES cell clones will require screening, so it is important that a robust and easy PCR strategy is used – it is for this reason that it is usual to amplify the region across the short arm of homology (Figure 14.6). Positive clones must then be further analysed, usually by Southern blotting and DNA sequencing, to verify that all regions of the targeting vector have undergone the desired recombination event.
Step 4. Injection of ES cells into Blastocysts
Blastocysts, which are 3.5-day-old embryos, are collected from the uterus of the donor female. It is usual when using ES cells from the 129 strain of mouse to collect blastocysts from a C57Bl/6 mother; this mouse line has a black coat colour (which is helpful when identifying chimeric mice – see step 5 below). ES cells carrying the desired mutation are treated to give a single cell suspension. The ES cells are drawn up into the injection pipette by gentle suction and the blastocyst to be injected is held by suction on the holding pipette. The injection pipette is advanced into the cavity of the blastocyst, which is known as the blastocoel, and 10–15 ES cells are released (Figure 4). After injection, the embryos are cultured for a few hours to allow them to re-expand slowly before being transferred to the uterus of a pseudo-pregnant foster mother. Pups should be born 17 days later.
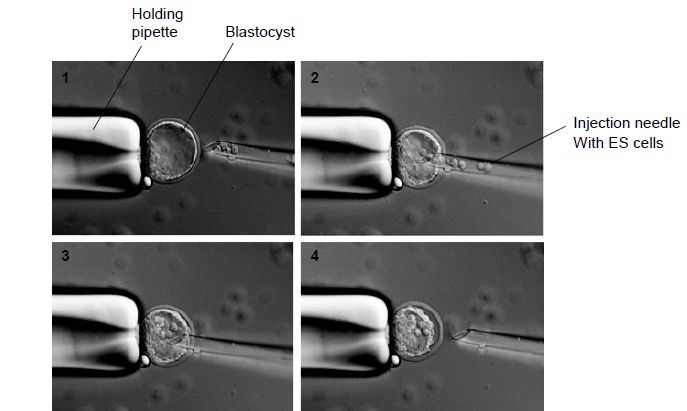
Figure 4. Injection of targeted ES cells into blastocysts. The blastocyst is held on the holding pipette by gentle suction (1). The injection needle containing ES cells is advanced into the blastocyst cavity (blastocoel) (2), where the ES cells are released (3) and the injection needle is removed (4).
Step 5. Identification of Chimeric Mice and Breeding to Generate Homozygous Mutant (Knockout) Mice Approximately 1 week after mouse pups are born, their coat colour becomes apparent. At this stage, it is possible to identify agouti from non-agouti coat colour. It is therefore possible to identify chimeric mice by their coat colour if ES cells from the 129 mouse strain (agouti) have contributed to the development of a C57Bl/6 embryo (non-agouti).
Embryos in which the ES cells had made no contribution would appear as wild-type C57Bl/6 (black), whereas those pups in which the 129 ES cells had made a contribution would contain a certain level of agouti coat colouring. Chimeric mice therefore contain some cells carrying the targeted mutation on one allele and other cells which are wild type. To generate a gene knockout mouse, it is essential that some of the germ cells carry the targeted mutation. To test for germline transmission of the mutation, chimeric mice are bred to wild-type mice; should germline transmission occur, a proportion of the pups will be heterozygous for the targeted mutation. Heterozygous mice can then be bred to produce mice homozygous for the targeted mutation – gene knockout mice.
Summary of Advantages and Disadvantages of Generating Gene Knockout Mice
As will be evident from the section above, the generation of a knockout mouse by gene targeting in ES cells is not a process to be undertaken lightly. It is labour intensive, technically demanding and fraught with problems; however, with good experimental design and know-how, this technique will provide enormous amounts of invaluable information about the function of the target gene.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|