


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 9-11-2020
Date: 7-3-2016
Date: 20-3-2016
|
PARTICLE-WAVE NATURE OF MATTER
Noting the wave and particle nature of light, de Broglie proposed that the electron had both a wave and a particle nature. While electrons had clearly exhibited a particle behavior in various experiments, de Broglie suggested that it was the wave nature of the electron that was responsible for the special allowed orbits in Bohr’s theory. De Broglie presented a simple wave picture where, in the allowed orbits, an integer number of wavelengths fit around the orbit. Orbit 1 had one wavelength, orbit 2 had two wavelengths, etc. In De Broglie’s picture, electron waves in non allowed orbits would cancel themselves out. Borrowing some features of Einstein’s photon theory of light waves, de Broglie could show that the angular momentum of the electron would have the special quantized values when the electron wave was in one of the special, non cancelling orbits.
With his simple wave picture, de Broglie had hit upon the fundamental idea that was missing in classical physics. The idea is that all matter, not just light, has a particle wave nature.
It took a few years to gain a satisfactory interpretation of the dual particle wave nature of matter. The current interpretation is that things like photons are in fact particles, but their motion is governed, not by Newtonian mechanics, but by the laws of wave motion. How
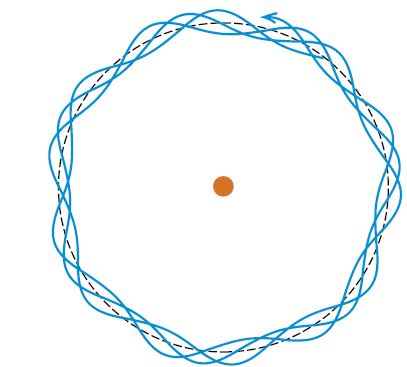
Figure 1: De Broglie picture of an electron wave cancelling itself out.
this works in detail is the subject of our chapter on Quantum Mechanics. One fundamental requirement of our modern interpretation of the particle wave is that, for the interpretation to be meaningful, all forms of matter, without exception, must have this particle wave nature. This general requirement is summarized by a rule discovered by Werner Heisinberg, a rule known as the uncertainty principle. How the rule got that name is also discussed in our chapter on quantum mechanics.
In 1925, after giving a seminar describing de Broglie’s model of electron waves in hydrogen, Erwin Schrödinger was chided for presenting such a “childish” model. A colleague reminded him that waves do not work that way, and suggested that since Schrödinger had nothing better to do, he should work out a real wave equation for the electron waves, and present the results in a couple of weeks.
It took Schrödinger longer than a couple of weeks, but he did succeed in constructing a wave equation for the electron. In many ways Schrödinger’s wave equation for the electron is analogous to Maxwell’s wave equation for light. Schrödinger’s wave equation for the electron allows one to calculate the behavior of electrons in all kinds of atoms. It allows one to explain and predict an atom’s electron structure and chemical properties. Schrödinger’s equation has become the fundamental equation of chemistry.
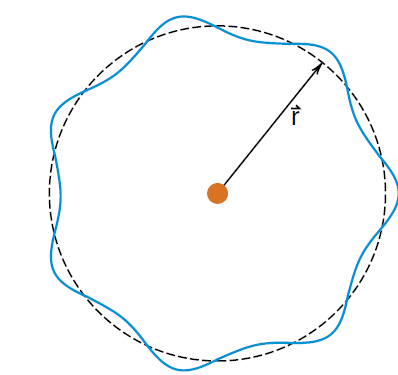
Figure 2: If the circumference of the orbit is an integer number of wavelengths, the electron wave will go around without any cancellation.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|