


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-2-2016
Date: 24-11-2019
Date: 3-12-2015
|
The reactivity of aromatic pi bonds in SEAr reactions is very sensitive to the presence of electron-donating groups (EDG) and electron-withdrawing groups (EWG) on the aromatic ring. This is due to the carbocation nature of the intermediate, which is stabilized by electron-donating groups and destabilized by electron-withdrawing groups.
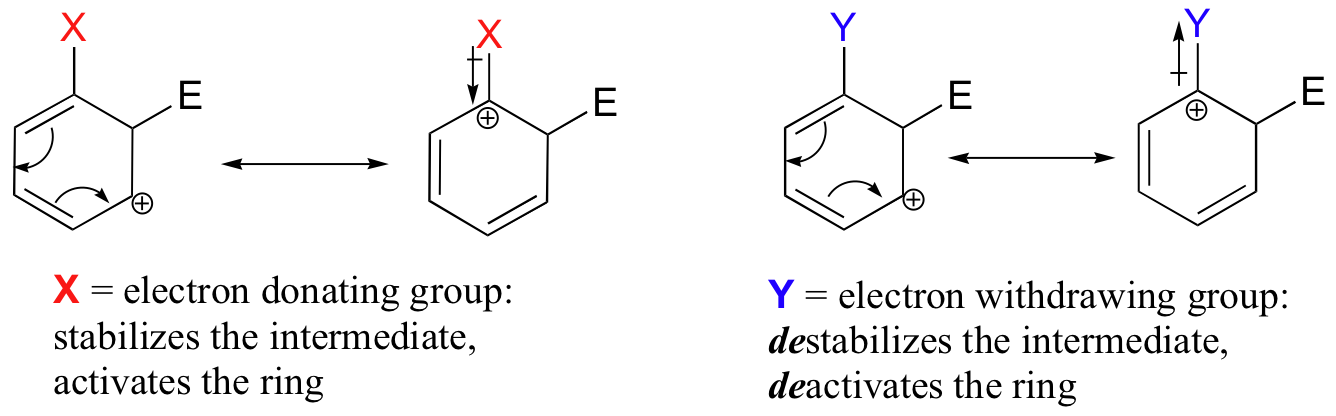
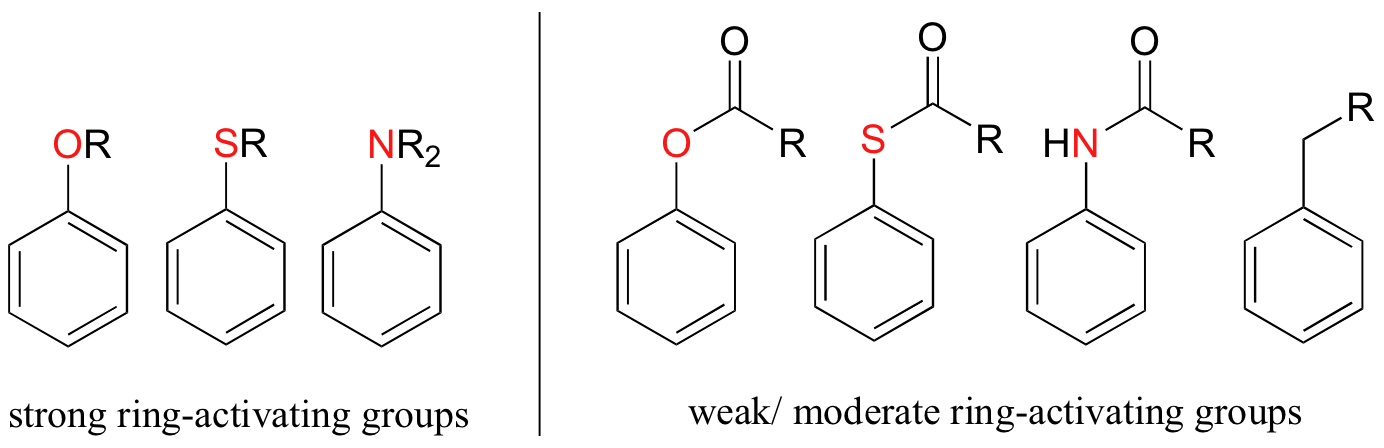
Alkyl groups are weakly ring-activating groups, as their electron-donating ability stems only from weak inductive effects. Substituents with heteroatoms connected to the aromatic ring are significantly more ring-activating than alkyl groups, because resonance electron-donating effects are possible. Amines, for example, are very powerful ring-activating substituents, due to the ability of the lone pair on the nitrogen to stabilize the carbocation intermediate through resonance:

Other ring-activating groups are shown below (in these figures, the R group can be a hydrogen). All of these groups are able, in varying degrees, to stabilize the carbocation intermediate in an electrophilic aromatic substitution reaction. Notice that plain old alkyl groups are also (weakly) ring-activating.
Substituent groups that are ring-activating due to resonance effects also tend to exert a strong regiochemical influence on further substitution reactions. Specifically, substitution tends to occur in the ortho and para positions relative to the existing group. This is known as the ortho-para directing effect. The effect can be explained by drawing resonance contributors for the carbocation intermediate of the SEAr reaction: the positive charge is in position to be delocalized by resonance only in reactions leading to ortho or para substitution.
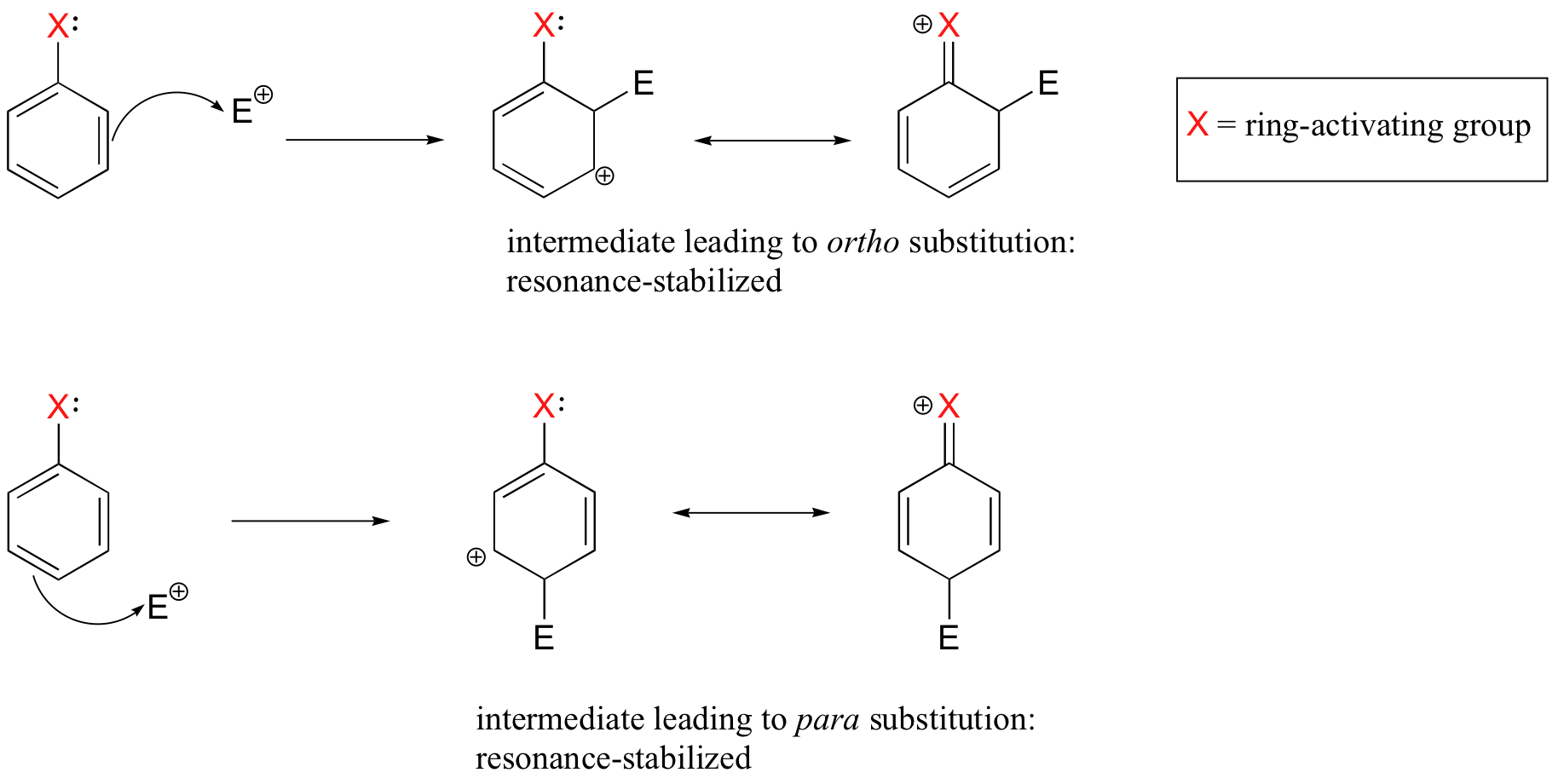
The carbocation which leads to the meta-substituted product, however, cannot be stabilized by resonance with the ring-activating group:

As an example, the Friedel-Crafts alkylation of methoxy benzene would be expected to produce a mixture of the ortho and para substituted products, but no meta-substituted product.
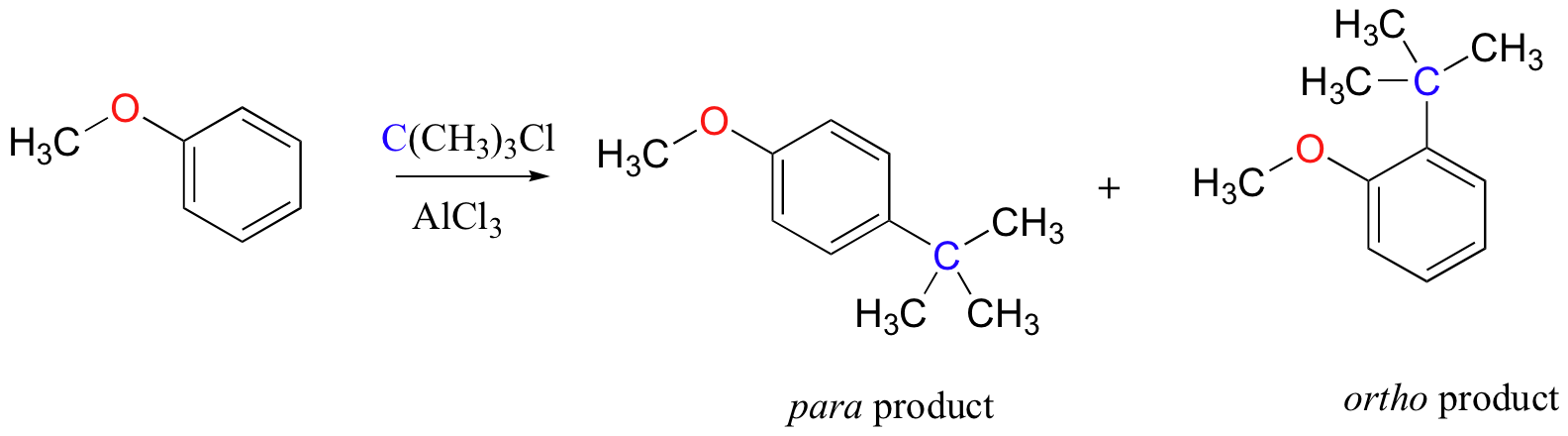
In addition, the para product would be expected to be preferred over the ortho product, due to steric considerations.
Electron-withdrawing substituents on an aromatic ring are ring-deactivating, making it harder for further substitution reactions to occur. These are mostly carbonyl-containing groups, as well as alkyl halides.
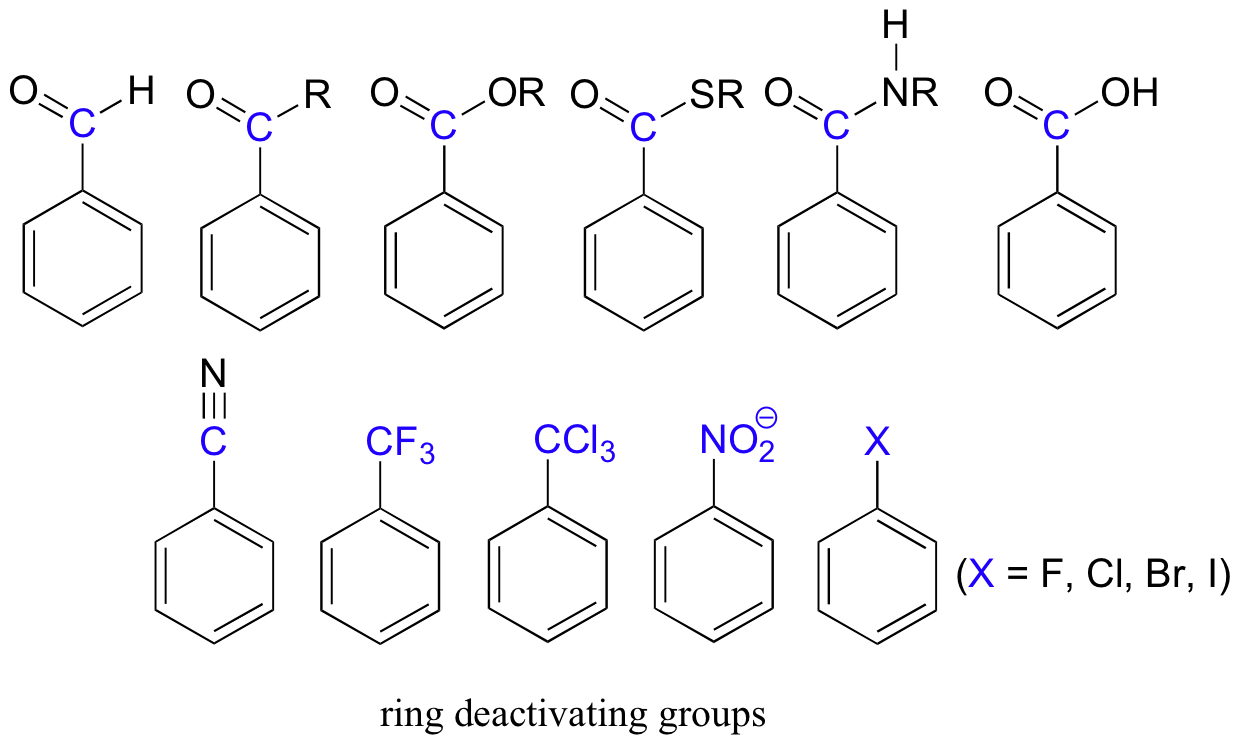
When substitution does occur on an aromatic ring with deactivating group already attached, it tends to occur specifically at the meta position – deactivating groups are generally meta-directing. The exception to this rule is the halogens, which are ring-deactivating but ortho-para directing (see next section).



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
علي بابا تطلق نماذج "Qwen" الجديدة في أحدث اختراق صيني لمجال الذكاء الاصطناعي مفتوح المصدر
|
|
|
|
|
|
|
مشاتل الكفيل تنتج أنواعًا مختلفة من النباتات المحلية والمستوردة وتواصل دعمها للمجتمع
|
|
|