


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-8-2018
Date: 7-8-2019
Date: 3-1-2020
|
Butane shows two different peaks in the 13C NMR spectrum, below. Note that: the chemical shifts of these peaks are not very different from methane. The carbons in butane are in a similar environment to the one in methane.
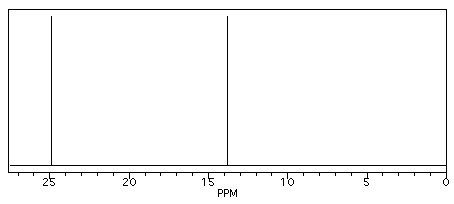
Figure NMR2. Simulated 13C NMR spectrum of butane (showing only the upfield portion of the spectrum).
In the 13C NMR spectrum of pentane (below), you can see three different peaks, even though pentane just contains methyl carbons and methylene carbons like butane. As far as the NMR spectrometer is concerned, pentane contains three different kinds of carbon, in three different environments. That result comes from symmetry.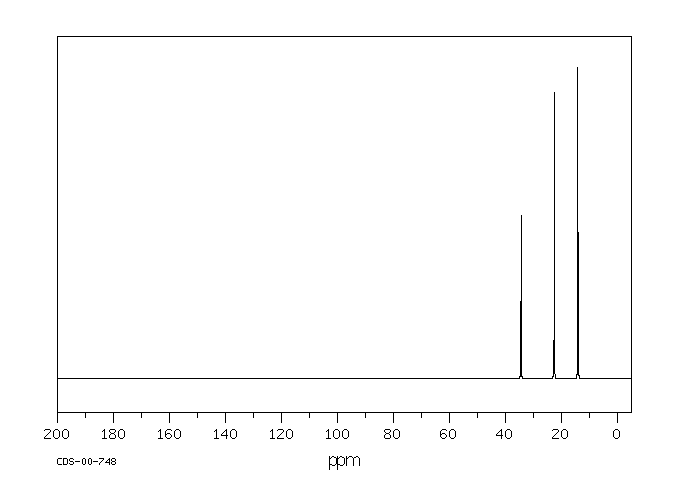
Figure NMR3.13C NMR spectrum of pentane. Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
Symmetry is an important factor in spectroscopy. Nature says:
To learn about symmetry, take a model of pentane and do the following:
Animation NMR1. A three-dimensional model of pentane. Grab the model with the mouse and rotate it so that you are convinced that the second and fourth carbons are symmetry-equivalent, but the third carbon is not.
By the same process, you can see that the second and fourth carbons along the chain are also symmetry-equivalent. However, the middle carbon is not; it never switches places with the other carbons if you rotate the model. There are three different sets of inequivalent carbons; these three groups are not the same as each other according to symmetry.



|
|
|
|
لمكافحة الاكتئاب.. عليك بالمشي يوميا هذه المسافة
|
|
|
|
|
|
|
تحذيرات من ثوران بركاني هائل قد يفاجئ العالم قريبا
|
|
|
|
|
|
|
العتبة العباسية تشارك في معرض النجف الأشرف الدولي للتسوق الشامل
|
|
|