


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-4-2019
Date: 21-2-2019
Date: 17-4-2019
|
Empirical formulas give the relative numbers of the different elements in a sample of a compound, expressed in the smallest possible integers. The term empirical refers to the fact that formulas of this kind are determined experimentally; such formulas are also commonly referred to as empirical formulas.
Example : Empirical formula from molecular formula
Glucose (the "fuel" your body runs on) is composed of molecular units having the formula C6H12O6. What is the empirical formula of glucose?
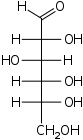
SOLUTION
The glucose molecule contains twice as many atoms of hydrogen as carbons or oxygens, so we divide through by 6 to get CH2O.
Note: this empirical formula, which applies to all 6-carbon sugars, indicates that these compounds are "composed" of carbon and water, which explains why sugars are known as carbohydrates.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|