
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-7-2020
Date: 5-5-2019
Date: 19-12-2015
|
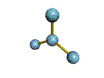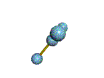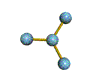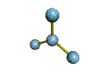 |
AX3 |
 |
Shape: | trigonal planar |
| Steric Number: | 3 | |
| Lone Pairs: | 0 | |
| Polar/NonPolar: | NonPolar | |
| Hybridization: | sp2 | |
| Examples: | BF3, CO32- |
NOTES: This molecule is made up of 3 equally spaced sp2 hybrid orbitals arranged at 120o angles. The shape of the orbitals is planar triangular. Since there is an atom at the end of each orbital, the shape of the molecule is also planar triangular.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|