
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Molecularity and Order are Identical for Elementary Reactions or Steps |
|
|
|
Read More
Date: 18-12-2020
Date: 22-9-2018
Date: 17-12-2020
|
Molecularity and Order are Identical for Elementary Reactions or Steps
The rate of an elementary reaction is proportional to the number of collisions between molecules (or atoms) of reactions. The number of collisions in turn is proportional to the concentration of each reactant molecule (or atom). Thus for a reaction.
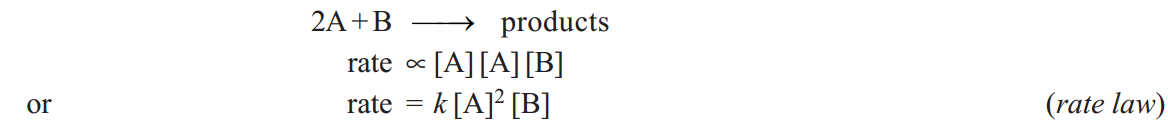
Two molecules of A and one molecule of B are participating in the reaction and, therefore, molecularity of the reaction is 2 + 1 = 3. The sum of powers in the rate law is 2 + 1 and hence the reaction order is also 3. Thus the molecularity and order for an elementary reaction are equal.
TABLE 1.1. MOLECULARITY AND ORDER FOR ELEMENTARY REACTIONS.
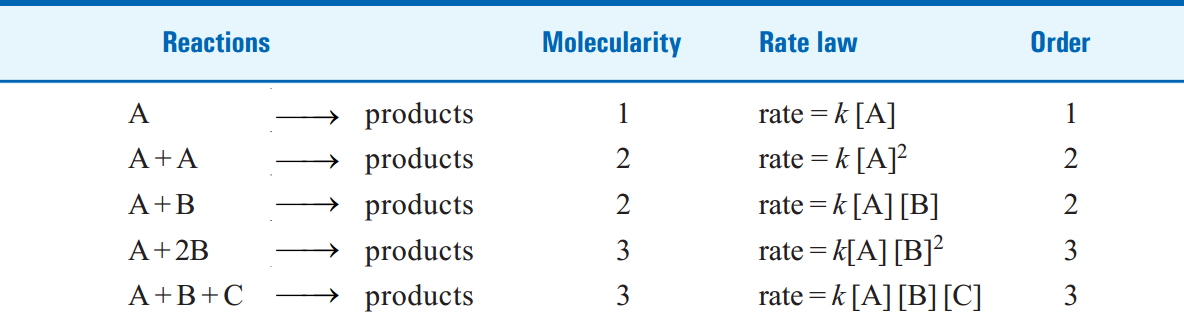



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|