


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-7-2017
Date: 28-9-2018
Date: 19-7-2017
|
Average Rate of Reaction is a Function of Time
Let us consider the reaction between carbon monoxide (CO) and nitrogen dioxide.
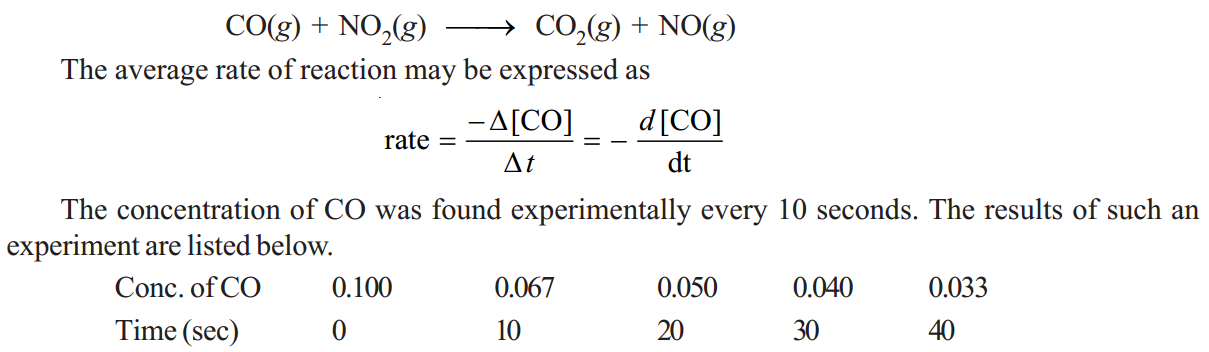
The results are also shown graphically in below.
As the reaction proceeds the concentration of CO decreases rapidly in the initial stages of the reaction. Then the concentration of CO decreases more and more slowly. Obviously the rate of reaction is a function of time.
Over the first 10 seconds, the average rate is
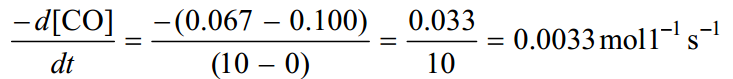
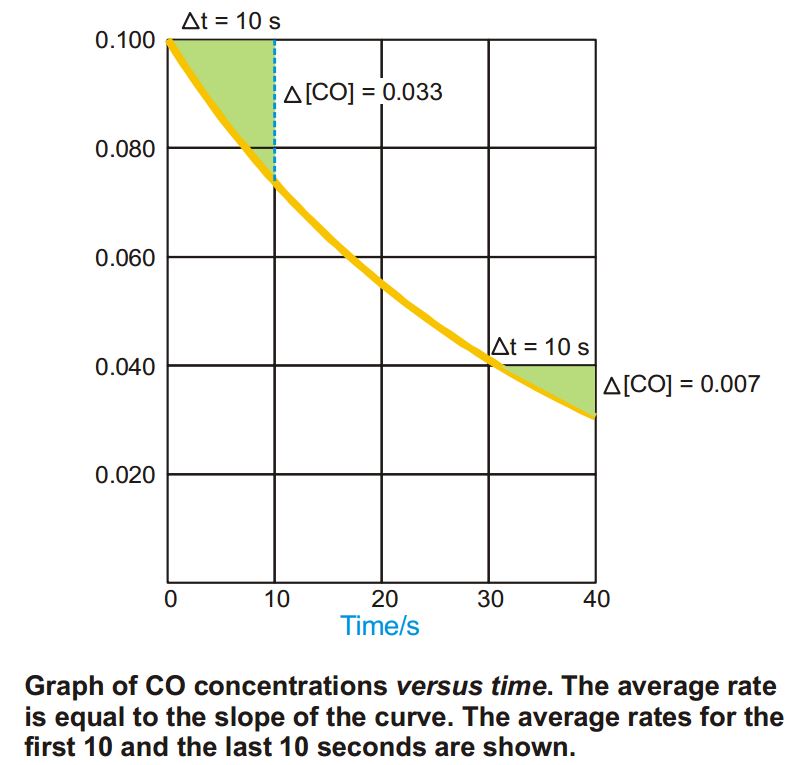
In the time interval between 30 and 40 seconds, the average rate is much smaller.
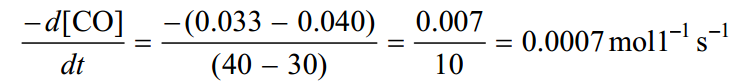
The reaction in indeed slowing down with time.
We shall see that average rates are not always useful. They cover a large time interval during which the rate of reaction changes significantly. So, a better way to estimate the rate of reaction, we need to make the time interval as small as possible.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|