


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-7-2019
Date: 11-9-2018
Date: 22-7-2019
|
Several useful and general procedures for degrading carboxylic acid derivatives to amines all involve the rearrangement of an acyl nitrene to an isocyanate. Although the nitrogen atom of a nitrene has no formal charge, it is electron deficient and serves as a locus for 1,2-rearrangements. As illustrated in the following diagram, acyl nitrenes may be generated from different amide-like starting compounds. Once formed, acyl nitrenes quickly rearrange to relatively stable isocyanate isomers, which may be isolated or reacted with hydroxylic solvents. The most common application of this rearrangement is for the synthesis of amines. Thus, addition of water to the ketene-like isocyanates produces an unstable carbamic acid that decomposes to an amine and carbon dioxide. General procedures for obtaining the nitrene precursors are listed below the diagram.

Hofmann Route: Primary amides are converted to N-halogenated derivatives by the action of HOX or X2 in alkaline solution. Excess base generates a conjugate base of the product.
Lossen Route: A hydroxamic acid derivative (RCONHOH) is made by reacting an ester with hydroxyl amine. The hydroxamic acid is O-acylated and then converted to its conjugate base.
Curtius Route: An acyl azide (RCON3) is prepared in one of two ways. (i) Reaction of an acyl chloride with sodium azide, or (ii) Reaction of an ester with excess hydrazine, followed by reaction of the acylhydrazide product (RCONHNH2) with cold nitrous acid. Acyl azides decompose to isocyanates on heating.
Schmidt Route: A variant of the Curtius procedure in which a carboxylic acid is heated with hydrazoic acid (HN3) and an acid catalyst.
Some examples of these different rearrangements are shown in the following diagram. In each case a green capitol letter (C, H, L or S) designates the type of reaction. The first three reactions illustrate the Hofmann rearrangement, which is a particularly useful method for preparing amines. The last example shows a Curtius reaction applied to a diester by way of an intermediate bis-acylhydrazide. An alternative Curtius approach to the amine product of example # 2 is also shown. The Curtius procedure is particularly useful if the isocyanate is the desired product, since the decomposition of the acyl azide intermediate can be conducted in the absence of hydroxylic solvents that would react with the isocyanate.
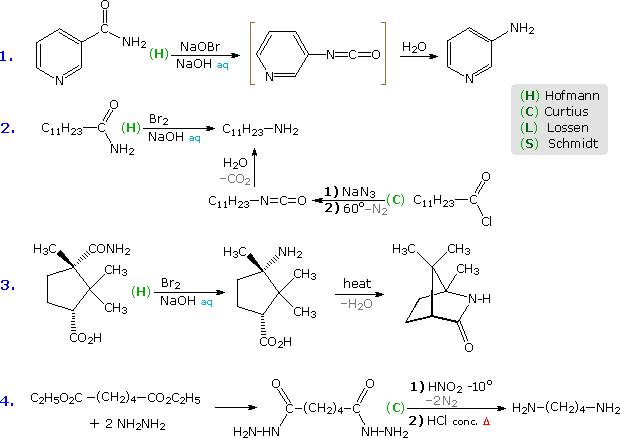

Example # 5 shows a Schmidt reaction in which an optically active carboxylic acid is the substrate. The S-configuration of the migrating phenethyl group is retained in the amine product, confirming the intramolecular character of these rearrangements. Retention of configuration is also observed in Curtius, Lossen and Hofmann rearrangements of this kind. Reactions # 6 & # 7 are interesting cases in which water is absent during the formation and reaction of the isocyanate. The alcohol solvent in # 6 adds to the isocyanate to produce a carbamate ester, known as a urethane. Unlike the unstable carbamic acids, urethanes do not decompose and may be isolated as pure compounds. If water had been the solvent, the resulting 1º-enamine would have rearranged to an imine and hydrolyzed to an aldehyde. In the Lossen rearrangement (# 7) butyl amine adds to the isocyanate to give a substituted urea (urea is NH2CONH2).
The Hofmann rearrangement in reaction # 8 provides a novel example of the tautomerism of an acetylenic 1º-amine to a nitrile. Finally, the last example illustrates a selective Hofmann rearrangement of a bromo-imide. The reactivity of the carbonyl group para to the electron withdrawing nitro substituent is increased relative to the other imide carbonyl. Consequently, base-catalyzed hydrolysis takes place there preferentially, leaving the acyl nitrene moiety meta to the nitro function.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|