


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-7-2019
Date: 12-10-2020
Date: 20-7-2019
|
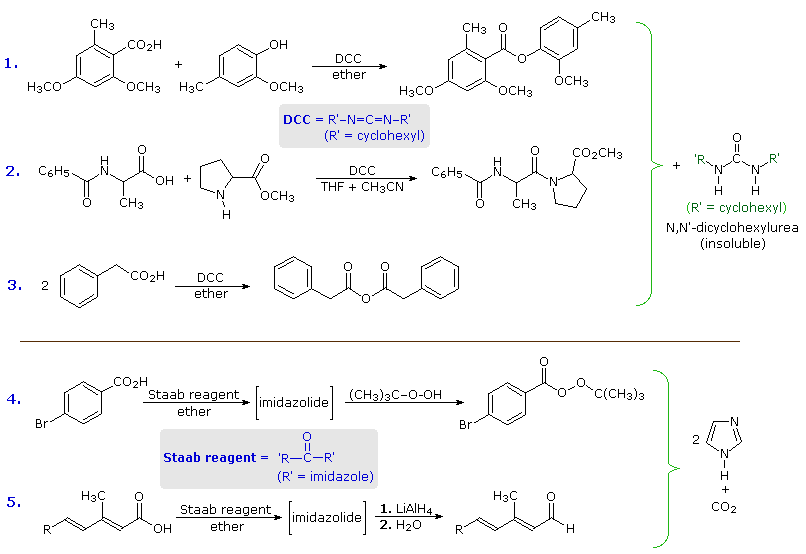
The ideal acylating reagent would be a carboxylic acid, but the acids themselves are relatively unreactive with nucleophiles. A simple solution to this inactivity, as noted earlier, was to convert the carboxylic acid to a more reactive derivative such as an acyl chloride or anhydride. A less extreme alternative procedure, often used in difficult cases, makes use of reagents which selectively activate a carboxyl group toward nucleophilic substitution. Two such reagents are dicyclohexylcarbodiimide (DCC) and carbonyldiimidazole (Staab's reagent). The following equations provide examples of their use in the preparation of esters, amides, anhydrides and peresters. Indeed, LAH reduction of the imidazolide intermediate generated by the Staab reagent provides a useful preparation of aldehydes from acids.
Keep in mind that imidazole is a stronger acid than water and a better leaving group than hydroxide anion, especially if protonated.
Mechanisms




|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|