


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-9-2018
Date: 28-8-2018
Date: 17-9-2018
|
Ambiguity in determining the initial site of carbocation formation presented a problem in the analysis of many pinacol rearrangements. This uncertainty can be removed by nitrous acid deamination of the corresponding 1º-aminoalcohols, as shown in the following equation. Since this reaction is normally carried out under very mild conditions, the possibility that subsequent transformations may obscure the initial rearrangement is reduced considerably.

The Tiffeneau-Demjanov rearrangement is often used to transform a cyclic ketone into a homologue that is one ring size larger. Such an application, which proceeds by way of a cyanohydrin intermediate, is shown in the first example below. Cyclic ketones have two alpha-carbon atoms, each of which might shift to the nascent 1º-carbocation. If R = H in the case shown here, these two groups are identical and on shifting give the same product. If R = CH3, the 2º-alkyl group shifts preferentially, the chief product being 3-methylcyclohexanone; the 2-methyl isomer is a minor product. The second reaction is informative because it demonstrates that the chiral 2º-butyl group moves with retention of configuration.
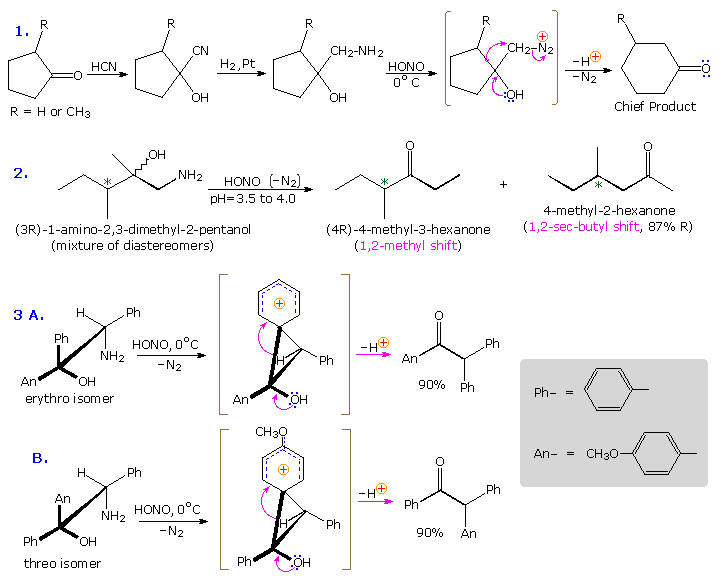
The third example illustrates the importance of substrate configuration on the course of rearrangement. The initial stage of an aryl group shift to an adjacent carbocation site may be viewed as an intramolecular electrophilic substitution of the Friedel-Crafts type. Aryl ring approach from the side opposite to the departing nitrogen of the diazonium ion generates a phenonium ion intermediate (shown in brackets above), the structure of which is similar to a benzenonium ion. In these two examples, diastereomeric reactants lead preferentially to diastereomeric intermediates, even though the anisyl group has a much greater migratory aptitude than phenyl. Electron pair donation by the hydroxyl substituent then acts to open the three-membered ring of these intermediates, yielding the ketone products.



|
|
|
|
مخاطر عدم علاج ارتفاع ضغط الدم
|
|
|
|
|
|
|
اختراق جديد في علاج سرطان البروستات العدواني
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|