

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Hydrogen and Halogen Atom Abstraction
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
27-8-2018
2791
Hydrogen and Halogen Atom Abstraction
If free radical reactions are to be useful to organic chemists, methods for transferring the reactivity of the simple radicals generated by the previously described homolysis reactions to specific sites in substrate molecules must be devised. The most direct way of doing this is by an atom abstraction, as shown here.
R–H + X• –––> R• + H–X
Indeed, when X is Cl or Br, this is a key step in the alkane halogenation chain reaction. Hydrogen abstraction reactions of this kind are sensitive to the nature of both the attacking radical ( X•) and the R–H bond. This is illustrated by the relative rates of hydrogen abstraction given in the following table. Each horizontal row of data is normalized to 1º C–H (1.0), but there are also large differences between rows. Thus the rate of reaction of 1º C–H with Cl• is a thousand times faster than with Br•. However, the less reactive bromine atom shows much greater selectivity in discriminating between 1º, 2º and 3º C–H groups.
Certain C–H bonds are so susceptible to radical attack that they react with atmospheric oxygen (a diradical) to form peroxides. Typical groups that exhibit this trait are 3º-alkyl, 2º & 3º-benzyl and alkoxy groups in ethers.
R–H + O2 –––> R• + •O2H –––> R–O–O–H
The exceptional facility with which S–H and Sn–H react with alkyl radicals makes thiophenol and trialkyltin hydrides excellent radical quenching agents, when present in excess. At equimolar or lower concentration they function well as radical transfer agents..
Relative Reactivities (per hydrogen) of Hydrogen Atom Donors with Selected Radicals
|
Hydrogen Donor |
C2H6 |
RCH2R |
R3CH |
C6H5CH3 |
CH2=CH-CH2R |
RCOCH3 |
C6H5SH |
(C4H9)3SnH |
|---|---|---|---|---|---|---|---|---|
|
Attacking Radical |
||||||||
|
CH3• |
1.0 | 10 | 100 | 85 | 25 | 170 | 4•107 | 5•105 |
|
(CH3)3CO• |
1.0 | 6 | 15 | 10 | 0.5 | 3.5 | -- | 104 |
|
Cl• |
1.0 | 5 | 6 | 2 | -- | -- | -- | -- |
|
Br• |
1.0 | 220 | 2•104 | 5•104 | -- | -- | -- | -- |
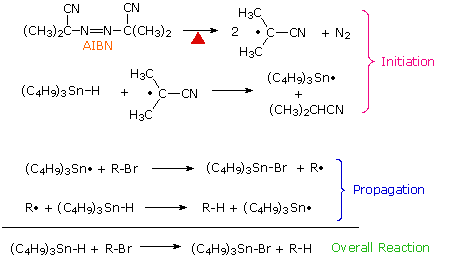
Carbon halogen bonds, especially C–Br and C–I, are weaker than C–H bonds and react with alkyl and stannyl radicals to generate new alkyl radicals. This reaction has been put to practical use in a mild procedure for reducing alkyl halides to alkanes. The chain reaction sequence that accomplishes this reduction is shown here. By clicking on the diagram, three examples of this dehalogenation reaction will be displayed.

An important modification of this reduction is shown in the third example above. The use of equimolar amounts of tributyltin hydride in reactions presents certain problems, including the toxicity presented by organostannanes, difficulty in separating nonpolar stannanes, such as halides, bis(tributyltin) and bis(tributyltin) oxide from desired products, and formation of tin oxides by reaction with moisture. To reduce these difficulties, a catalytic amount of the stannanes may be used together with enough NaBH4 (or an equivalent reagent) to convert the tributyltin halides to the hydride. Indeed, the reduction is so facile that traces of peroxides in the reactants often initiate reaction without added AIBN.
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















