

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Cationic Polymerization
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
19-1-2022
3687
Cationic Polymerization
Polymerization of an alkene by acidic reagents can be formulated by a mechanism similar to the addition of hydrogen halides to alkene linkages. First, a proton from a suitable acid adds to an alkene to yield a carbocation. Then, in the absence of any other reasonably strong nucleophilic reagent, another alkene molecule donates an electron pair and forms a longer-chain cation. Continuation of this process can lead to a high-molecular-weight cation. Termination can occur by loss of a proton. The following equations represent the overall reaction sequence:
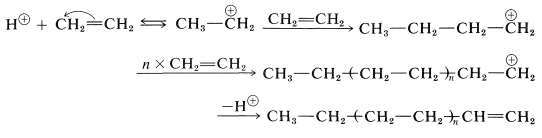
Ethene does not polymerize by the cationic mechanism because it does not have sufficiently electron-donating groups to permit easy formation of the intermediate growing-chain cation. 2-Methylpropene has electron-donating alkyl groups and polymerizes much more easily than ethene by this type of mechanism. The usual catalysts for cationic polymerization of 2-methylpropene are sulfuric acid, hydrogen fluoride, or a complex of boron trifluoride and water. Under nearly anhydrous conditions a very long chain polymer called polyisobutylene is formed.
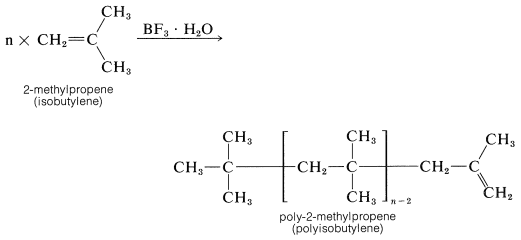
Polyisobutylene fractions of particular molecular weights are very tacky and are used as adhesives for pressure-sealing tapes.
In the presence of 60% sulfuric acid, 2-methylpropene is not converted to a long-chain polymer, but to a mixture of eight-carbon alkenes. The mechanism is like that of the polymerization of 2-methylpropene under nearly anhydrous conditions, except that chain termination occurs after only one 2-methylpropene molecule has been added:
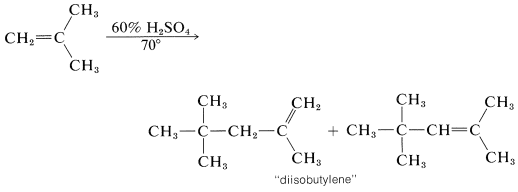
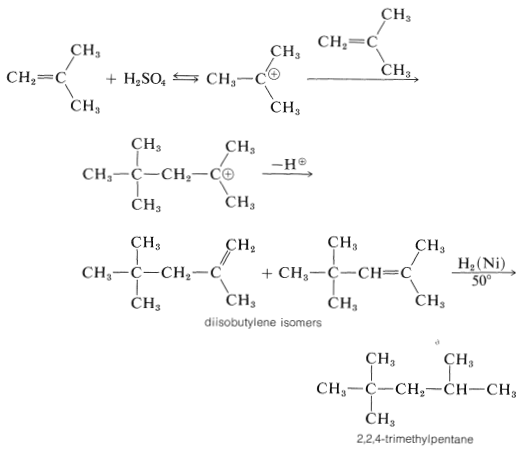
The short chain length is due to the high water concentration; the intermediate carbocation loses a proton to water before it can react with another alkene molecule.
The proton can be lost in two different ways, and a mixture of alkene isomers is obtained. The alkene mixture is known as "diisobutylene" and has a number of commercial uses. Hydrogenation yields 2,2,4-trimethylpentane (often erroneously called "isooctane"), which is used as the standard "100 antiknock rating" fuel for internal-combustion gasoline engines:
 الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
الاكثر قراءة في المركبات الوسطية وميكانيكيات التفاعلات العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















