

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-5-2019
Date: 4-7-2017
Date: 3-1-2017
|
Hydrogen azide and azide salts
Sodium azide, NaN3, is obtained from molten sodium amide by reacting NaNH2 with NaNO3 at 450 Kand treatment of NaN3 with H2SO4 yields hydrogen azide, HN3.
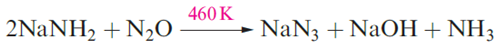
Hydrogen azide (hydrazoic acid) is a colourless liquid (mp 193 K, bp 309 K); it is dangerously explosive (ΔfHo(l, 298 K) = 264 kJ mol-1) and highly poisonous. Aqueous solutions of HN3 are weakly acidic.
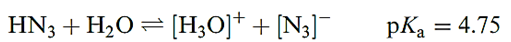
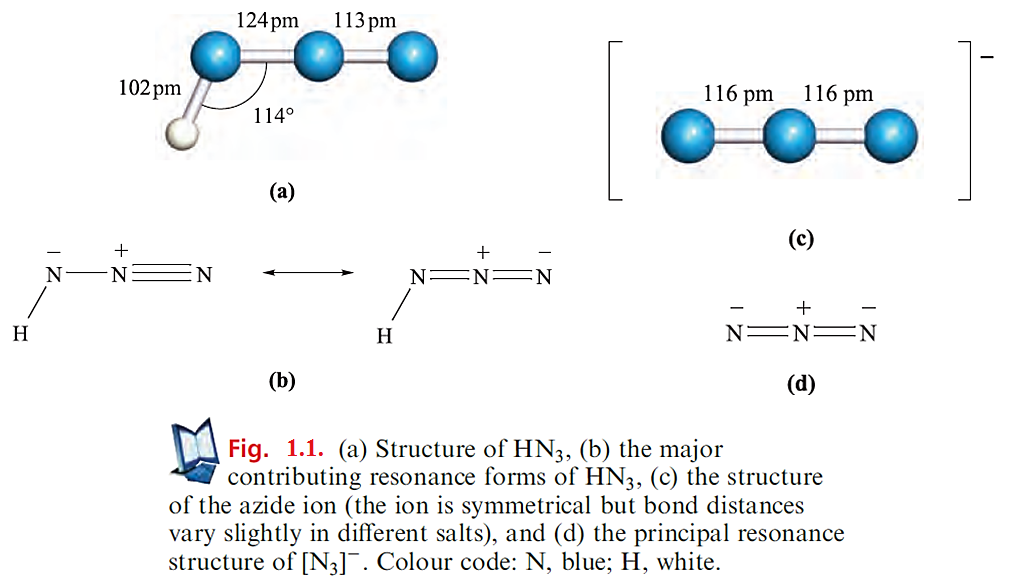
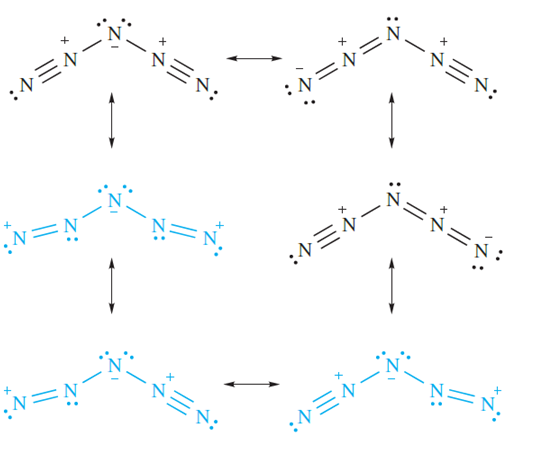
The structure of HN3 is shown in Figure 1.1a, and a consideration of the resonance structures in Figure 1.1b provides an explanation for the asymmetry of the NNN-unit. The azide ion is isoelectronic with CO2, and the symmetrical structure of [N3]- (Figure 1.1c) is consistent with the bonding description in Figure 1.1d. A range of azide salts is known; Ag(I), Cu(II) and Pb(II) azides, which are insoluble in water, are explosive, and Pb(N3)2 is used as an initiator for less sensitive explosives. On the other hand, group 1 metal azides decompose quietly when heated. The reaction between NaN3 and Me3SiCl yields the covalent compound Me3SiN3 which is a useful reagent in organic synthesis. Reaction below occurs when Me3SiN3 is treated with [PPh4][N3] - in the presence of ethanol.
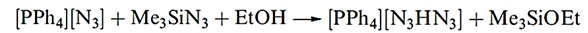
The [N3HN3]- anion in the product is stabilized by hydrogen bonding (compare with [FHF]- . Although the position of the H atom in the anion is not known with great accuracy, structural parameters for the solid state structure of [PPh4][N3HN3] (Figure 1.2) are sufficiently accurate to confirm an asymmetrical N_H……N interaction (N……N = 272 pm).
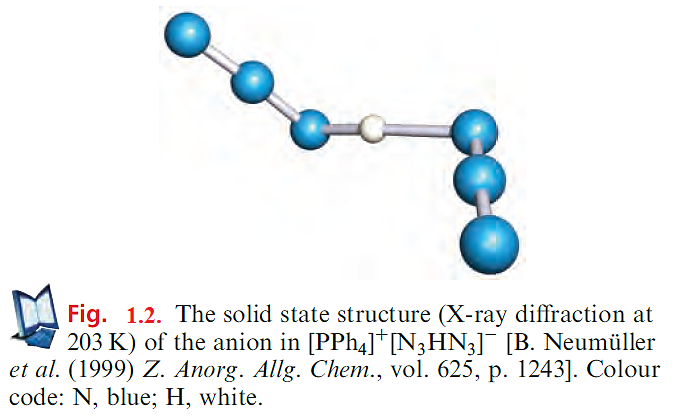
The azide group, like CN. (though to a lesser extent), shows similarities to a halogen and is another example of a pseudohalogen. However, no N6 molecule (i.e. a dimer of N3- and so an analogue of an X2 halogen) has yet been prepared. Like halide ions, the azide ion acts as a ligand in a wide variety of metal complexes, e.g. [Au(N3)4]-, trans-[TiCl4(N3)2]2-, cis-[Co(en)2(N3)2], trans-[Ru(en)2(N2)(N3)] (which is also an example of a dinitrogen complex, Figure 1.3a) and [Sn(N3)6]2- (Figure 1.3b).
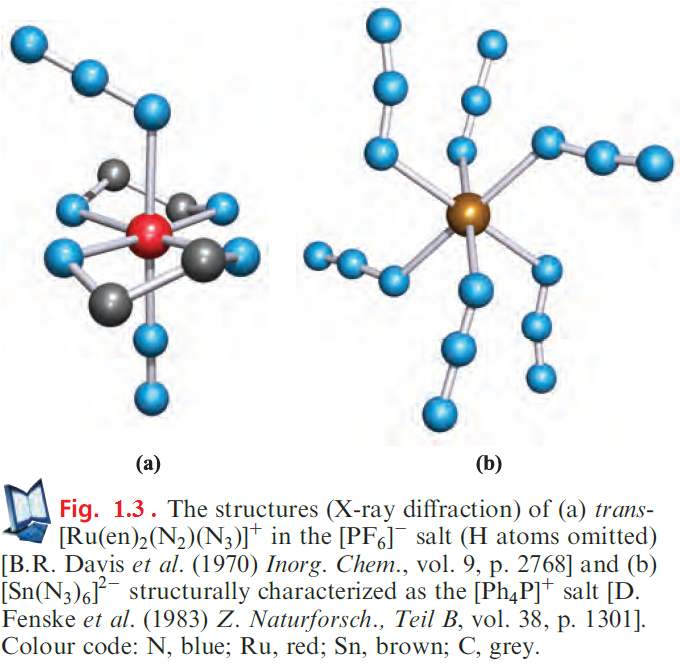
The reaction of HN3 with [N2F][AsF6] in HF at 195K results in the formation of [N5][AsF6]. Designing the synthesis of [N5]+ was not trivial. Precursors in which the N≡N and N=N bonds are preformed are critical, but should not involve gaseous N2 since this is too inert. The HF solvent provides a heat sink for the exothermic reaction, the product being potentially explosive. Although [N5][AsF6] was the first example of a salt of [N5]+ and is therefore of significant interest, it is not very stable and tends to explode. In contrast, [N5][SbF6] is stable at 298K and is relatively resistant to impact. Solid [N5][SbF6] oxidizes NO, NO2 and Br2 (scheme below), but not Cl2 or O2.
The reaction of [N5][SbF6] with SbF5 in liquid HF yields [N5][Sb2F11], the solid state structure of which has been determined, confirming a V-shaped [N5]+ ion (central N_N_N angle =1118). The N_N bond lengths are 111pm (almost the same as in N2) and 130pm (slightly more than in MeN=NMe), respectively, for the terminal and central bonds. Resonance stabilization is a key factor in the stability of [N5]+ and provides a degree of multiple-bond character to all the N_N bonds.
The three resonance structures shown in blue contain one or two terminal sextet N atoms. Their inclusion helps to account for the observed Nterminal_N_Ncentral bond angles of 1688.
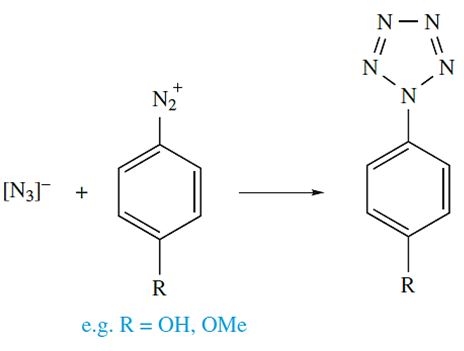
The reaction of sodium azide with aryldiazonium salts yields arylpentazoles (equation below), from which it has been possible to generate the cyclic anion [N5]- through molecular fragmentation in an electrospray ionization mass spectrometer.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|