


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-1-2019
Date: 28-3-2017
Date: 22-12-2018
|
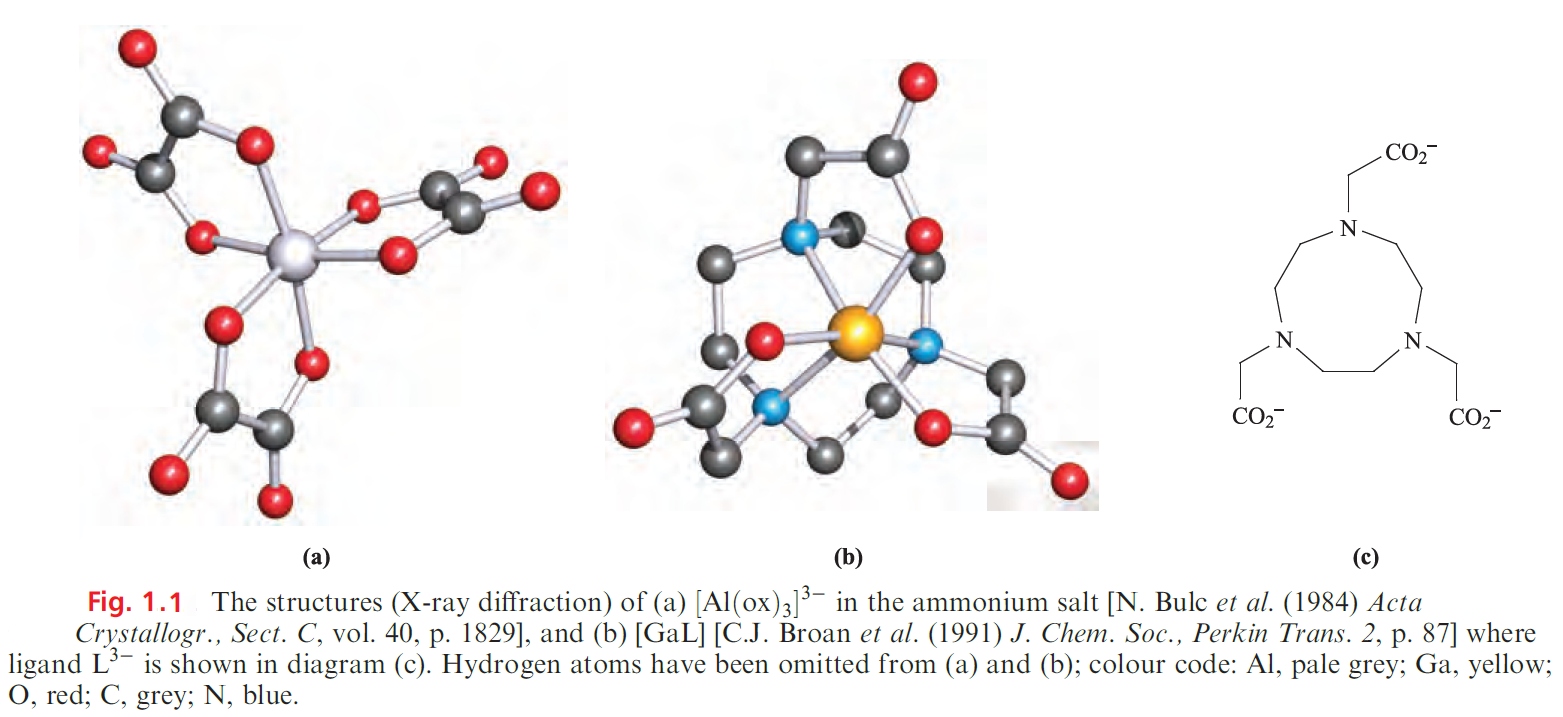
Coordination complexes of the M3+ ions
Increasing numbers of coordination complexes of the group 13 metal ions are now known. Octahedral coordination is common, e.g. in [M(acac)3] (M = Al, Ga, In), [M(ox)3]3- (M = Al, Ga, In) and mer-[Ga(N3)3(py)3]. Figure 1.1a shows the structure of [Al(ox)3]3-. The complexes[M(acac)3] are structurally related to [Fe(acac)3] so we discussed the influence of [H]+ on the formation of [Fe(acac)3] and similar arguments apply to the group 13 metal ion complexes.

Deprotonation of 8-hydroxyquinoline gives the didentate ligand which has a number of applications. For example, Al3+ may be extracted into organic solvents as the octahedral complex [Al(8-hydroxyquinoline)3] providing a weighable form of Al3+ for the gravimetric analysis of aluminium.
Complexes involving macrocyclic ligands with pendant carboxylate or phosphate groups have received attention in the development of highly stable metal complexes suitable for in vivo applications, e.g. tumour-seeking complexes containing radioisotopes . The incorporation of 67Ga (gemitter, t1/2 = 3.2 days), 68Ga ( β+-emitter, t1/2 = 68 min) or 111 In (g-emitter, t1/2 = 2.8 days) into such complexes yields potential radiopharmaceuticals. Figure 1.1c shows an example of a well-studied ligand which forms very stable complexes with Ga(III) and In(III) (logK ≥ 20). The way in which this ligand encapsulates the M3+ ion with the three N-donor atoms forced into a fac-arrangement can be seen in Figure 1.1b.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
بالصور: معهد نور الإمام الحسين (ع) للمكفوفين وضعاف البصر التابع للعتبة الحسينية.. جهود كبيرة وخدمات متعددة
|
|
|